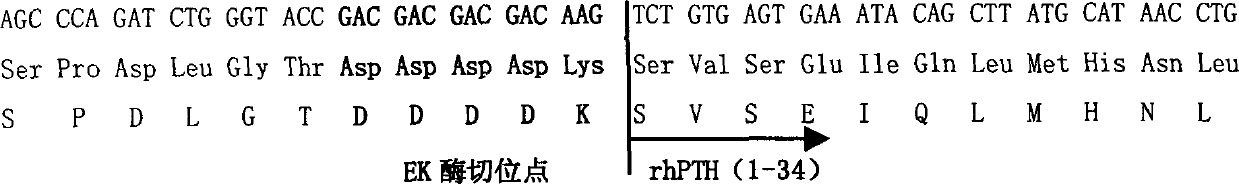
Recombinant human parathormone PTH1-34 preparation method
A technology of parathyroid hormone and enterokinase enzyme, which is applied in the fields of parathyroid hormone, hormone peptide, recombinant DNA technology, etc., can solve the problems of unsatisfactory expression purity and physiological activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Construction of Trx-PTH1-34 expression vector and host cell
[0071] See Figure 1 for the preparation process.
[0072] (a) Preparation of PTH1-34
[0073] Two primers were designed and synthesized, corresponding to PTH 1-8 amino acid residues and 29-34 amino acid residues, respectively, for PCR cloning of human PTH 1-34 cDNA. The primer sequences are as follows:
[0074] 5' primer:
[0075] 5′ATCTG GGTACCGACGACGACGACAAG TCTGTGAGTGAAATACAGCTTAT 3' (SEQ ID NO: 5)
[0076] 3' Primer:
[0077] 5'TTATCAAAAATTGTGCACATCCTGC 3' (SEQ ID NO: 6)
[0078] The 5' primer contains the enterokinase cleavage point DDDDK and the N-terminal gene sequence of PTH (1-34), and introduces the KpnI cleavage point; the 3' primer contains the C-terminal gene sequence of PTH (1-34) and a stop codon. Using the above primers, PCR was carried out using conventionally prepared human parathyroid hormone cDNA as a template. Then PCR2.1-TOPO cloning kit of Invitrogen Company was used to directly...
Embodiment 2
[0084] Expression of Trx-PTH1-34
[0085] Pick the positive Escherichia coli B21(DE3) / Trx-PTH1-34 on the transformation plate, streak it on the agar plate of LB+1% glucose containing 100 μg / ml Amp, cultivate it at 37°C for 16 hours, and pick the single cells with good growth The colonies were inoculated in LB + 1% glucose culture solution (containing Amp 100 μg / ml) at 37° C., 270 rpm, and cultured overnight. The overnight bacteria were inoculated at 1:50 in LB+1% glucose culture solution (containing Amp 100 μg / ml), and cultured with shaking at 37°C. When OD 600 When it reaches 0.6-0.8, add IPTG to the final concentration of 1mM, and induce for 3 hours. The bacterial solution was centrifuged at 4000rpm for 10 minutes to remove the supernatant, the precipitated bacterial cells were added to the sample buffer, and the bacteria were broken in a water bath at 100°C for 5 minutes. After centrifugation, the soluble protein supernatant was subjected to SDS-PAGE electrophoresis (5% s...
Embodiment 3
[0089] Separation and purification of fusion protein and PTH1-34
[0090] (a) Separation and purification of fusion protein
[0091] After the fermentation of the engineered bacteria, the bacterial suspension was ultrasonically treated in an ice bath. After breaking the bacteria, it was centrifuged at 4°C and 9000rpm for 20min, and the Trx-EK-PTH fusion protein was mainly in the supernatant.
[0092] Purification by metal chelate column chromatography affinity column chromatography was performed as follows: 60 mM imidazole, 500 mM NaCl, pH 8.0, 20 mM PB loading buffer equilibrated for 3 column volumes. After the ultrasonic supernatant was filtered on a 0.45 μm membrane column, it was equilibrated with 60 mM imidazole, 500 mM NaCl, pH 8.0, and 20 mM PB buffer until the baseline was stable, and some impurities were removed. The fusion protein was eluted with 160mM imidazole, 500mM NaCl, pH8.0, 20mM PB buffer, and the peak of the fusion protein was collected and detected by SDS-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com