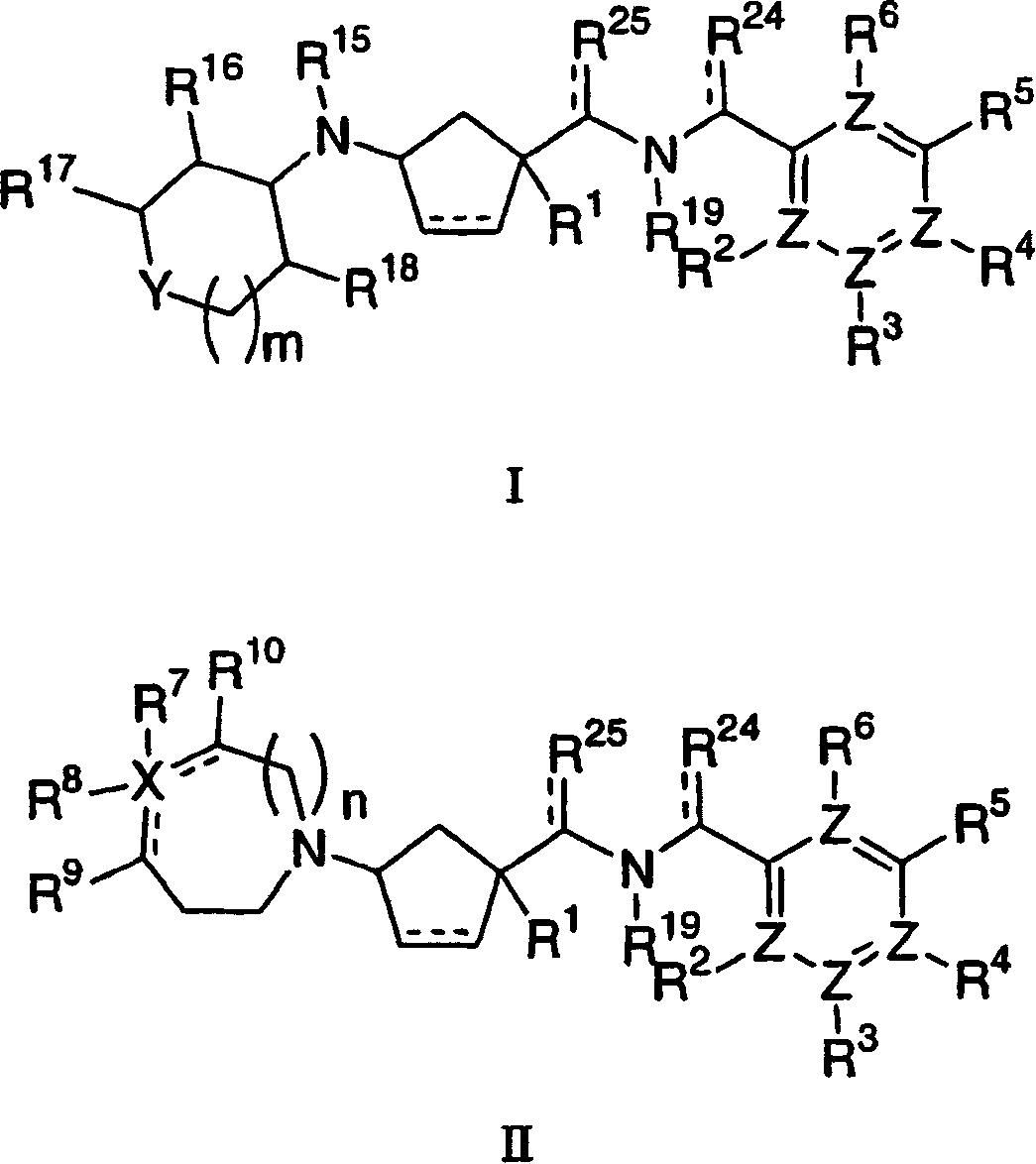
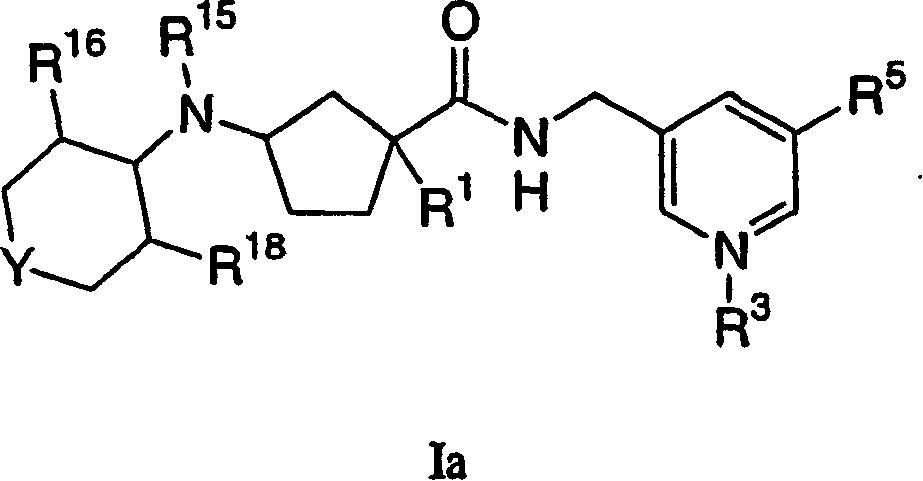
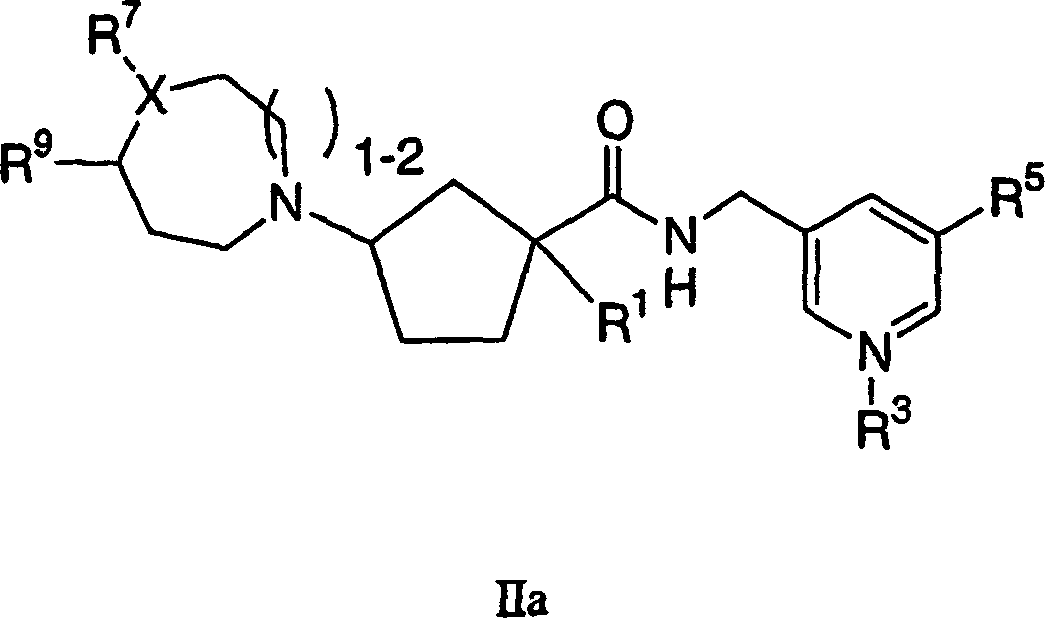
Tetrahydropyran heterocyclic cyclopentyl heteroaryl modulators of chemokine receptor activity
A technology of heterocycle and cycloalkyl is applied in the field of tetrahydropyran heterocyclopentyl heteroaryl modulation agent with chemokine receptor activity, which can solve the problems of low sensitivity of rheumatoid arthritis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0295]
[0296] Step A
[0297]
[0298] To a solution of Intermediate 2 (500 mg, 1.84 mmol) in dichloromethane (25 mL) was added Intermediate 6 (358, 2.03 mmol), N,N-diisopropylethylamine (1.06 mL, 6.08 mmol), 1-Hydroxy-7-azabenzotriazole (276 mg, 2.03 mmol) and EDC (583 mg, 3.04 mmol) and the solution was stirred overnight. The mixture was extracted with dichloromethane, washed with water, dried over sodium sulfate and concentrated in vacuo. The crude product was purified by MPLC (0-60% ethyl acetate / hexanes). 4N hydrochloric acid was added and the solution was concentrated in vacuo to give the desired product (660 mg, 90%).
[0299] Step B
[0300]
[0301] To a solution of the product obtained in Step A (168 mg, 0.42 mmol) in dichloromethane (20 mL) was added tetrahydro-4H-pyran-4-one (50 mg, 0.50 mmol) and N,N-diisopropyl Ethylamine (260 uL, 1.5 mmol). After addition of molecular sieves (25 mg), sodium triacetoxyborohydride (1.06 g, 5.00 mmol) was added and ...
Embodiment 2
[0303]
[0304] Step A
[0305]
[0306] To a solution of Intermediate 2 (500 mg, 1.84 mmol) in dichloromethane (25 mL) was added Intermediate 6 (358, 2.03 mmol), N,N-diisopropylethylamine (1.06 mL, 6.08 mmol), 1-Hydroxy-7-azabenzotriazole (276 mg, 2.03 mmol) and EDC (583 mg, 3.04 mmol), and the solution was stirred overnight. The mixture was extracted with dichloromethane, washed with water, dried over sodium sulfate and concentrated in vacuo. The crude product was purified by MPLC (0-60% ethyl acetate / hexanes). 4N hydrochloric acid was added, and the solution was concentrated in vacuo to give the desired product (660 mg, 90%).
[0307] Step B
[0308]
[0309] To a solution of the product from Step A (50 mg, 0.39 mmol) in dichloromethane (15 mL) was added intermediate 4 (128 mg, 0.32 mmol) and N,N-diisopropylethylamine (203 uL, 1.17 mmol). After addition of molecular sieves (15 mg), sodium triacetoxyborohydride (827 mg, 3.9 mmol) was added and the mixture was st...
Embodiment 3
[0311]
[0312] Step A
[0313]
[0314] To a solution of Intermediate 2 (500 mg, 1.84 mmol) in diazomethane (25 mL) was added Intermediate 6 (358, 2.03 mmol), N,N-diisopropylethylamine (1.06 mL, 6.08 mmol), 1-Hydroxy-7-azabenzotriazole (276 mg, 2.03 mmol) and EDC (583 mg, 3.04 mmol), and the solution was stirred overnight. The mixture was extracted with dichloromethane, washed with water, dried over sodium sulfate and concentrated in vacuo. The crude product was purified by MPLC (0-60% ethyl acetate / hexanes). 4N hydrochloric acid was added, and the solution was concentrated in vacuo to give the desired product (660 mg, 90%).
[0315] Step B
[0316]
[0317] To a solution of Intermediate 3 (17 mg, 0.150 mmol) in anhydrous dichloromethane (15 mL) was added the product of Step A (50 mg, 0.125 mmol) and N,N-diisopropylethylamine (65 uL, 0.375 mmol ). After addition of molecular sieves (10 mg), sodium triacetoxyborohydride (185 mg, 0.875 mmol) was added and the mixt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com