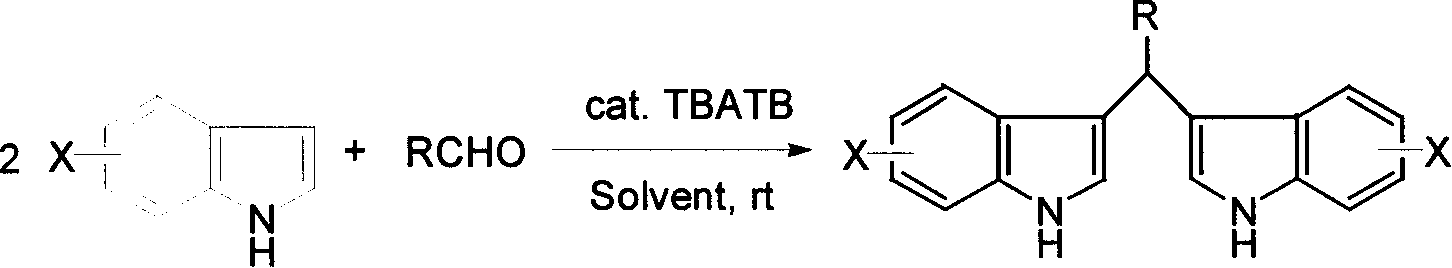
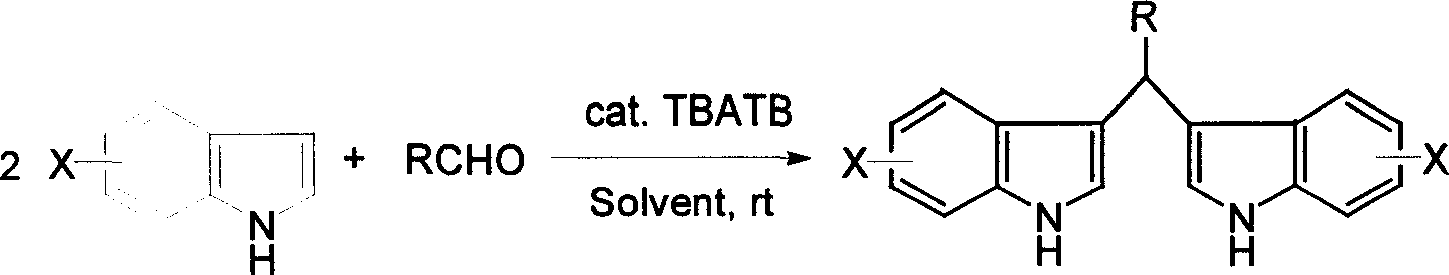
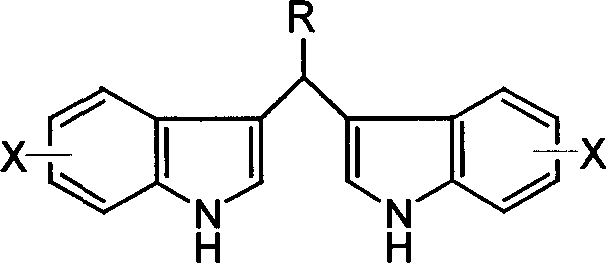
Process for preparing diindolylmethane derivatives
A technology of diindolylmethane and derivatives, applied in the field of indole synthesis, can solve the problems of low yield, strong acid conditions, expensive reagents and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 0.02 moles of indole and 0.01 moles of benzaldehyde were dissolved in 10 milliliters of methanol, then 0.5 millimoles of tetra-n-butylammonium tribromide was added, and the reaction was stirred and reacted at room temperature at 20° C. for 1 hour, and the reaction was terminated, and 20 milliliters of water was added to precipitate the product. After simple filtration and drying, bis(3-indole)phenylmethane was obtained with a yield of 90%.
[0023]
[0024] mp 123~125℃; IR(KBr) 3415, 1631, 1380, 734cm -1 ; 1 H NMR (500MHz, CDCl 3 )δ7.89 (brs, 2H), 7.38 (d, J=8.0Hz, 2H), 7.33-7.35 (m, 4H), 7.19-7.30 (m, 5H), 7.00 (m, 2H), 6.64 (d , J=1.0Hz, 2H), 5.88(s, 1H)ppm; 13 C NMR (125MHz, CDCl 3 )δ144.2, 136.8, 128.9, 128.4, 127.2, 126.3, 123.8, 122.1, 120.1, 119.8, 119.4, 111.2, 40.3ppm; MS m / z 322 (M + ); HRMS(EI)calcd for C 23 h 18 N 2 : 322.1470; Found 322.1473.
Embodiment 2
[0026] 0.02 moles of indole and 0.01 moles of p-methoxybenzaldehyde were dissolved in 10 milliliters of ethanol, then 0.4 millimoles of tetra-n-butylammonium tribromide was added, stirred and reacted at room temperature 25°C for 0.5 hours, the reaction was terminated, and 20 milliliters of water was added , the product was separated out, and bis(3-indolyl)-(4-methoxybenzene)methane was obtained after simple filtration and drying process with a yield of 94%.
[0027] OMe
[0028]
[0029] mp 179-181°C.IR,: 742, 1417, 1454, 1508, 1610, 2924, 3396cm -1 . 1 H NMR (400Hz, CDCl 3 ): 3.76(s, 3H), 5.80(s, 1H), 6.59(d, J=2.4Hz, 2H), 6.78(d, J=8.1Hz, 2H), 6.93(t, 2H), 7.12(t , 2H), 7.20-7.38 (m, 6H), 7.80 (brs, 2H, NH) ppm. 13 C-NMR (100MHz, CDCl 3 ): 39.2, 56.0, 110.8, 113.6, 118.6, 119.4, 121.8, 123.0, 126.4, 136.2ppm. EIMS: m / z (%): 352 (20, M + ), 236(100).
Embodiment 3
[0031] 0.021 moles of 5-methoxyindole and 0.01 moles of p-phenylbenzaldehyde were dissolved in 12 milliliters of methanol, then 0.6 millimoles of tetra-n-butylammonium tribromide was added, and the reaction was stirred at room temperature at 15° C. for 1.5 hours, and the reaction was completed. After adding 25 ml of water, the product was precipitated. After simple filtration and drying, bis(5-methoxy-3-indolyl)-(4-phenylbenzene)methane was obtained with a yield of 92%.
[0032]
[0033] mp 194-196°C; IR(KBr): 3470, 2965, 1270, 770cm -1 ; 1 H NMR (500MHz, CDCl 3 ): 3.72(s, 6H), 5.84(s, 1H), 6.69(d, J=1.7Hz, 2H), 6.86(m, 4H), 7.21-7.61(m, 11H), 7.81(br s, 2H , NH)ppm. 13 C NMR (125MHz, CDCl 3 ): 40.17, 56.10, 102.24, 111.97, 112.13, 119.36, 124.73, 127.15, 127.19, 127.24, 127.73, 128.96, 129.36, 132.11, 139.07, 141.30, 143.35, 14.z IME.8 (%) m + ).Anal.Calcd for C 31 h 26 N 2 o 2 : C, 81.20; H, 5.72; N, 6.11; Found: C, 81.20; H, 5.76; N, 6.14.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com