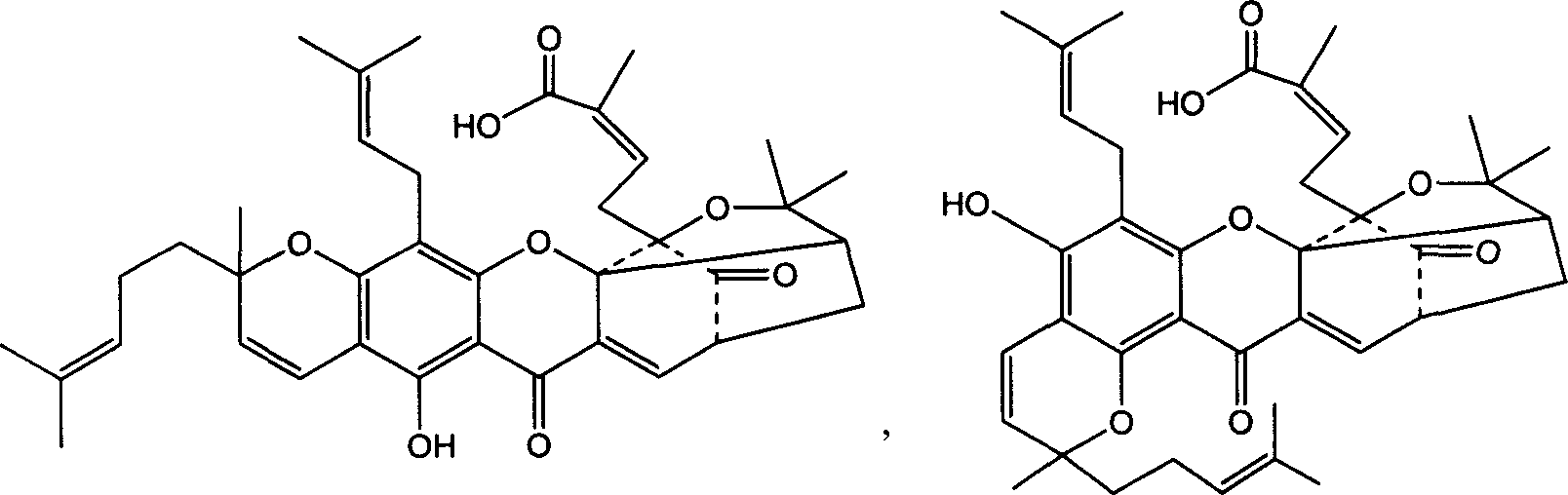
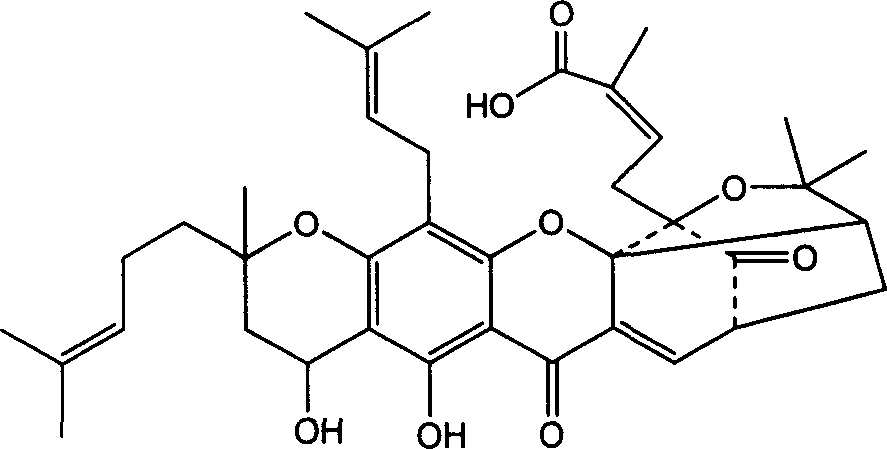
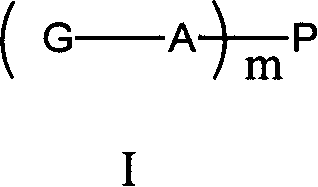Polyethylene glycol prodrug of gambogicacid, its preparation method, formulation and use
A technology for PEGylation of gambogic acid and polyethylene glycol is applied in the field of natural medicines and polymer materials, and can solve the problems of poor water solubility, limited clinical application, and high toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of Polyethylene Glycol 4K—Gambogic Acid
[0056] Weigh gambogic acid (0.346g, 0.55mmol), dicyclohexylcarbodiimide (DCC) (1.23g, 0.6mmol), p-dimethylaminopyridine (DMAP) (0.03g, 0.25mmol), dissolve in 30ml of dichloromethane was stirred at 0°C for 2h, PEG4K (1g, 0.25mmol) was added, the temperature was raised to 25°C, and stirred overnight. After filtration, the filtrate was concentrated to dryness, recrystallized from isopropanol, purified on a Sephadex G-25 dextran gel column, and freeze-dried to obtain 1.0 g of polyethylene glycol 4K-gambogic acid as a yellow powder, with a yield of 76.1%. mp: 52-54°C. Gambogic acid content: 21.93% (theoretical: 23.9%). Solubility in water at room temperature is about: 650mg / ml
[0057] 1 HNMR (500MHz, CDCl 3 ): Around δ3.6 is the strong peak of hydrogen on PEG, and the remaining peaks are peaks of hydrogen on gambogic acid, δ1.28-1.45 (C24-H, C25-H, 12H), δ1.55-2.06 (C29- H, C34-H, C35-H, C39-H, C40-H, 30H), δ2.31-2...
Embodiment 2
[0060] Preparation of polyethylene glycol 20K-glycine-gambogic acid
[0061] 1. Preparation of polyethylene glycol 20K-glycine trifluoroacetate (PEG20K-Gly-FA):
[0062] Weigh PEG20K (40g, 2mmol), N-tert-butoxycarbonylglycine (Boc-Gly) (0.77g, 4.4mmol), p-dimethylaminopyridine (DMAP) (0.12g, 1mmol), dissolve in 100ml dichloromethane , Another weighed dicyclohexylcarbodiimide (DCC) (1.2g, 4.8mmol), dissolved in 4ml of dichloromethane, and added dropwise to the reaction solution. Stir overnight at room temperature. Centrifuge, discard the precipitate, concentrate the clear liquid to dryness, add 40ml of cold acetone, filter again to remove a small amount of insoluble matter, concentrate the filtrate to dryness again, dissolve the residue in 20ml of dichloromethane, add dropwise to 300ml of vigorously stirred ice ether, and statically After the solid was completely precipitated, it was filtered and vacuum-dried to obtain 36.4 g of a white solid. Dissolve this white solid in 60...
Embodiment 3
[0068] Preparation of polyethylene glycol 10K-lysine-gambogic acid
[0069] 1. Preparation of polyethylene glycol-lysine trifluoroacetate (PEG10K-Lys-FA):
[0070] The operation method is the same as in Example 2, and the yield is 78.7%.
[0071] 2. Preparation of polyethylene glycol 10K-lysine-gambogic acid:
[0072] Weigh PEG-Lys-FA (1.25g, 0.125mmol), gambogic acid (0.47g, 0.75mmol), DCC (0.16g, 0.8mmol), triethylamine or 2ml dissolved in 20ml dichloromethane, stir at room temperature overnight. Filtrate, concentrate the filtrate to dryness, recrystallize from isopropanol, purify on a Sephadex G-25 dextran gel column, and lyophilize to obtain a yellow powder polyethylene glycol 20K-lysine-gambogic acid (PEG-Lys-GA ) 1.2g, yield: 68.3%. Gambogic acid content: 16.46% (theoretical: 19.79%). Solubility in water at room temperature is about 440mg / ml.
[0073] 1 HNMR (500MHz, CDCl 3 ): Around δ3.6 is the strong peak of hydrogen on PEG, and the remaining peaks are peaks of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com