Process for preparing copolymer containing biphenyl polyether ether-sulfone and poly(ether sulfone)
A technology of polyethersulfone terpolymer resin and biphenyl polyether, which is applied in the field of biphenyl-containing polyether ether sulfone and polyether sulfone terpolymer, and can solve the problem of low flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
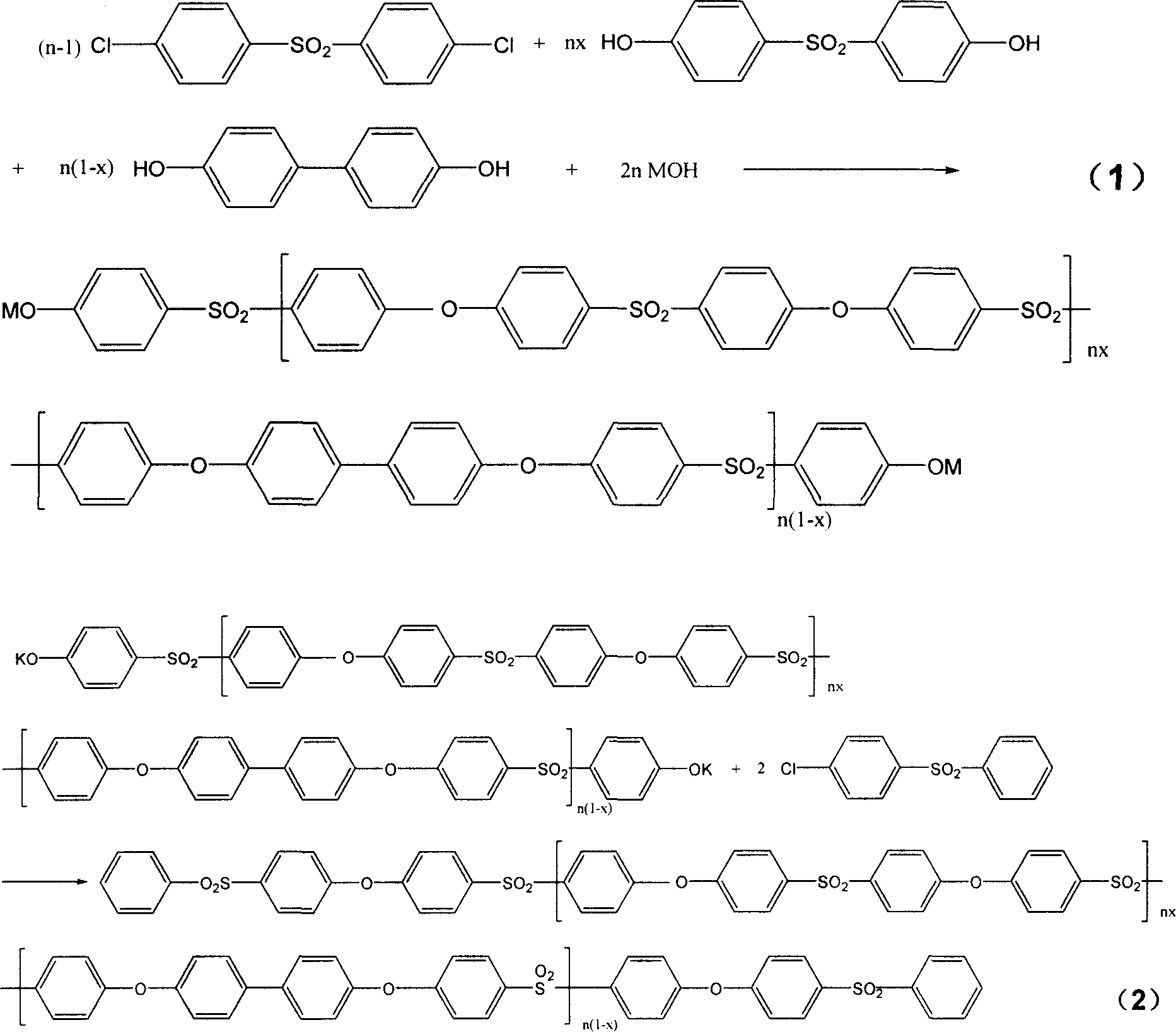
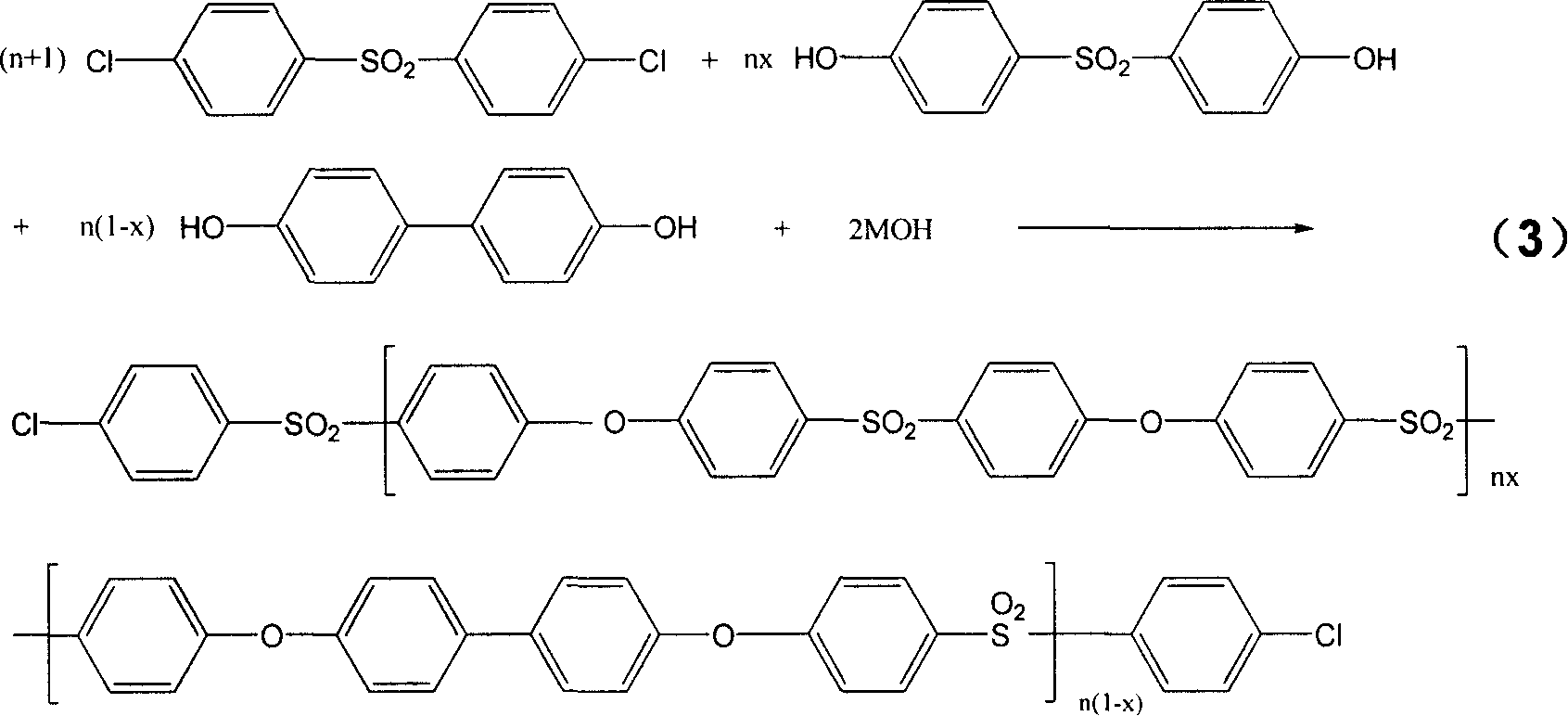
[0021] In a 1000ml three-necked reaction flask equipped with a thermometer, a nitrogen pipe, a condensing water separator, and a stirrer, first add 520g of refined sulfolane (dimethylsulfone, diphenylsulfone, etc. can also be used) and then sequentially add 142.87g (0.4975mol , with respect to biphenol and bisphenol-S molar number and 0.5mol deficiency 0.5% of the two chlorine ( ) and 0.93g (0.005mol) biphenol ( ) and 123.88g (0.495mol) bisphenol-S ( ) (the polymer generated by the reaction is 232 grams, and the solid content is about 31%), then add 78g dimethylbenzene (15% of the solvent quality) and stir, heat (the reaction bottle is placed in the electric heating mantle) to make the solid dissolve completely After continuing to heat to 80° C., add an alkali solution containing 56.11 g (1.0 mol) KOH, and the concentration of the alkali solution is 25%. Continue to heat up to 100°C and the system starts to azeotrope, xylene and water are condensed in the water separator,...
Embodiment 2
[0031] Change the batching ratio of Example 1, still get 520g refined sulfolane, then add 142.87g (0.4975mol, relative to biphenol, bisphenol-S molar number and 0.5mol less than 0.5%) of dichloro, The diphenol of 1.86g (0.01mol), the bisphenol-S of 122.63g (0.49mol), the add-on of xylene, KOH solution and monochlorsulfone and the addition condition are completely with embodiment 1, and according to All reactions were carried out under the same reaction conditions. After obtaining the polymer, carry out the refining process completely according to the same method and conditions as in Example 1 to obtain the benzene terminal product with X=0.98 as shown in the formula (2). The product that obtains obtains with identical method and condition test:
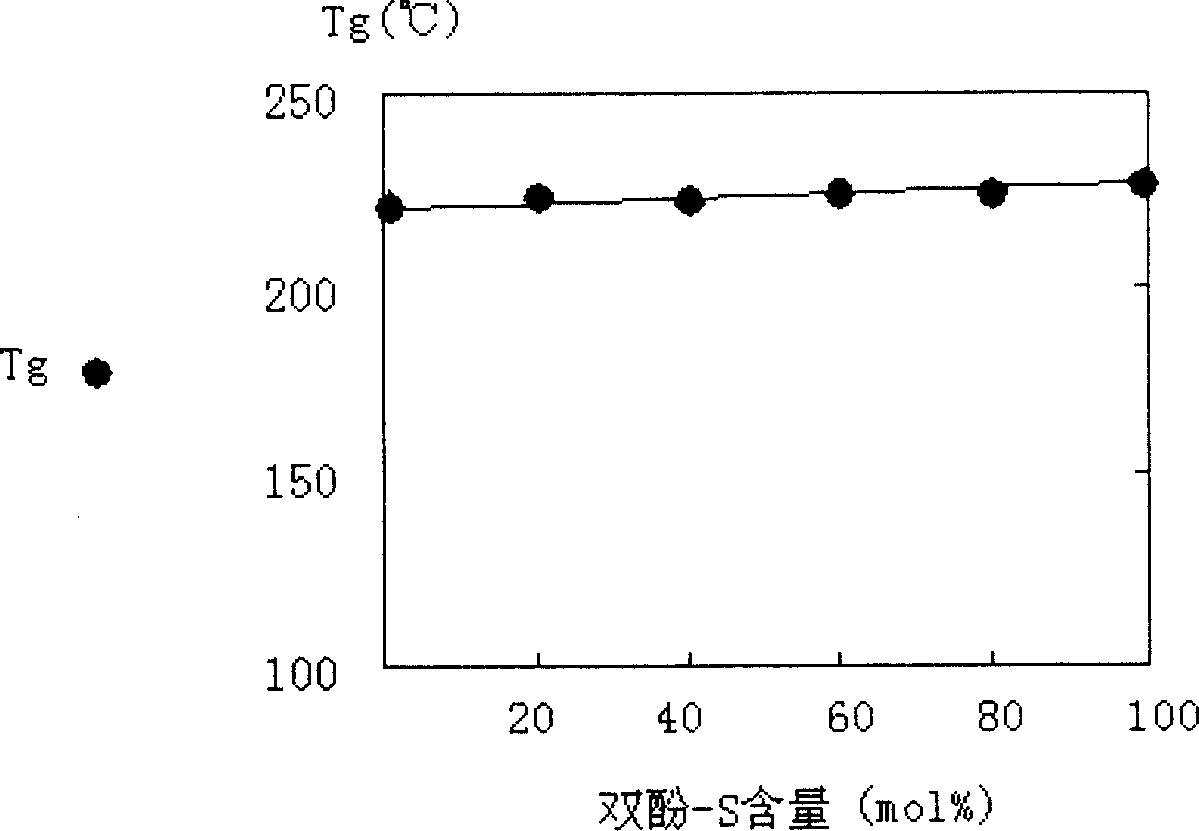
[0032] Tg=225℃
Embodiment 3
[0034] Change the batching ratio of Example 1, still get 520g refined sulfolane, then add 142.87g (0.4975mol, relative to biphenol, bisphenol-S molar number and 0.5mol less than 0.5%) of dichloro, The biphenyl diphenol of 4.65g (0.025mol), the bisphenol-S of 118.88g (0.475mol), the add-on and adding condition of xylene, KOH solution and monochlorsulfone are completely with embodiment 1 afterwards, and according to All reactions were carried out under the same reaction conditions. After obtaining the polymer, carry out the refining treatment completely according to the same method and conditions as in Example 1 to obtain the benzene terminal product with X=0.95 as shown in the formula (2). The product that obtains obtains with identical method and condition test:
[0035] Tg=225℃
[0036] B = 25%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com