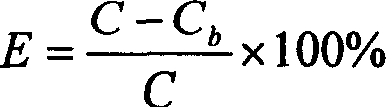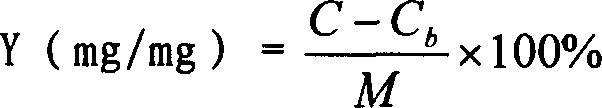Amphotericin B nano preparation
A technology of amphotericin and nano preparation, applied in the field of medicine, can solve the problems of high risk, many comorbidities, difficult to penetrate the blood-brain barrier, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 300mg of Dextran-70, put it in a beaker, add distilled water and stir to dissolve, adjust the pH value of the solution to 3.0 with 0.01mol / L hydrochloric acid, and set the volume to 50ml. Slowly add 0.5ml of BCA at room temperature under electromagnetic stirring to a final concentration of 1%. After stirring for 4 hours, adjust the pH to 8.5 with 0.1mol / L sodium hydroxide to obtain a blank PBCA nanoparticle colloidal solution. Measure 10ml of the blank nanoparticle colloidal solution, add 20mg of amphotericin B powder injection, measure the pH value to be about 7.5, and continue stirring for 4 hours to obtain the AmB-PBCA-NP colloidal solution. Finally, add 1% Tween-80 and incubate in a 37°C incubator for 2 hours to obtain the nano-preparation of the present invention. After testing, the encapsulation efficiency is 56.10%, the drug loading capacity is 82%, and the average particle size is 79.9nm.
Embodiment 2
[0027] Weigh 300mg of Dextran-70, put it in a beaker, add distilled water and stir to dissolve, adjust the pH value of the solution to 3.0 with 0.01mol / L hydrochloric acid, and set the volume to 50ml. Slowly add 0.5ml of BCA under electromagnetic stirring at room temperature to a final concentration of 1%. After stirring for 4 hours, adjust the pH to 7.5 with 0.1mol / L sodium hydroxide to obtain a blank PBCA nanoparticle colloidal solution. Measure 10ml of the blank nanoparticle colloidal solution, add 20mg of amphotericin B powder injection, and measure the pH value to be about 7.5, then add 20mg of cosolvent sodium deoxycholate, and continue stirring for 4 hours to obtain the AmB-PBCA-NP colloidal solution. Finally, add 1% Tween-80 and incubate in a 37°C incubator for 2 hours to obtain the nano-preparation of the present invention. After testing, the encapsulation efficiency is 23.1%, the drug loading capacity is 25%, and the average particle diameter is 90.7nm.
Embodiment 3
[0029] Weigh 300mg of Dextran-70 and 300mg of Poloxamer 188, put them in a beaker, add distilled water and stir to dissolve, adjust the pH value of the solution to 3.0 with 0.01mol / L hydrochloric acid, and dilute to 50ml. Slowly add 0.5ml of BCA at room temperature under electromagnetic stirring to a final concentration of 1%. After stirring for 4 hours, adjust the pH to 8.5 with 0.1mol / L sodium hydroxide to obtain a blank PBCA nanoparticle colloidal solution. Measure 10ml of the blank nanoparticle colloidal solution, add 20mg of amphotericin powder injection, measure the pH value to be about 7.0, and continue stirring for 4 hours to obtain the AmB-PBCA-NP colloidal solution. Finally, add 1% Tween-80 and incubate in a 37°C incubator for 2 hours to obtain the nano-preparation of the present invention. After testing, the encapsulation efficiency is 38.4%, the drug loading capacity is 92%, and the average particle diameter is 83.4nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com