Synthesis for producing levo phosphomycin by dextro phosphomycin
A technology for levofosfomycin and dextrofosfomycin, which is applied in the synthesis field of preparing levofosfomycin from dextrofosfomycin, can solve the problems of danger, inability to use in industrial production, difficulty in detection and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
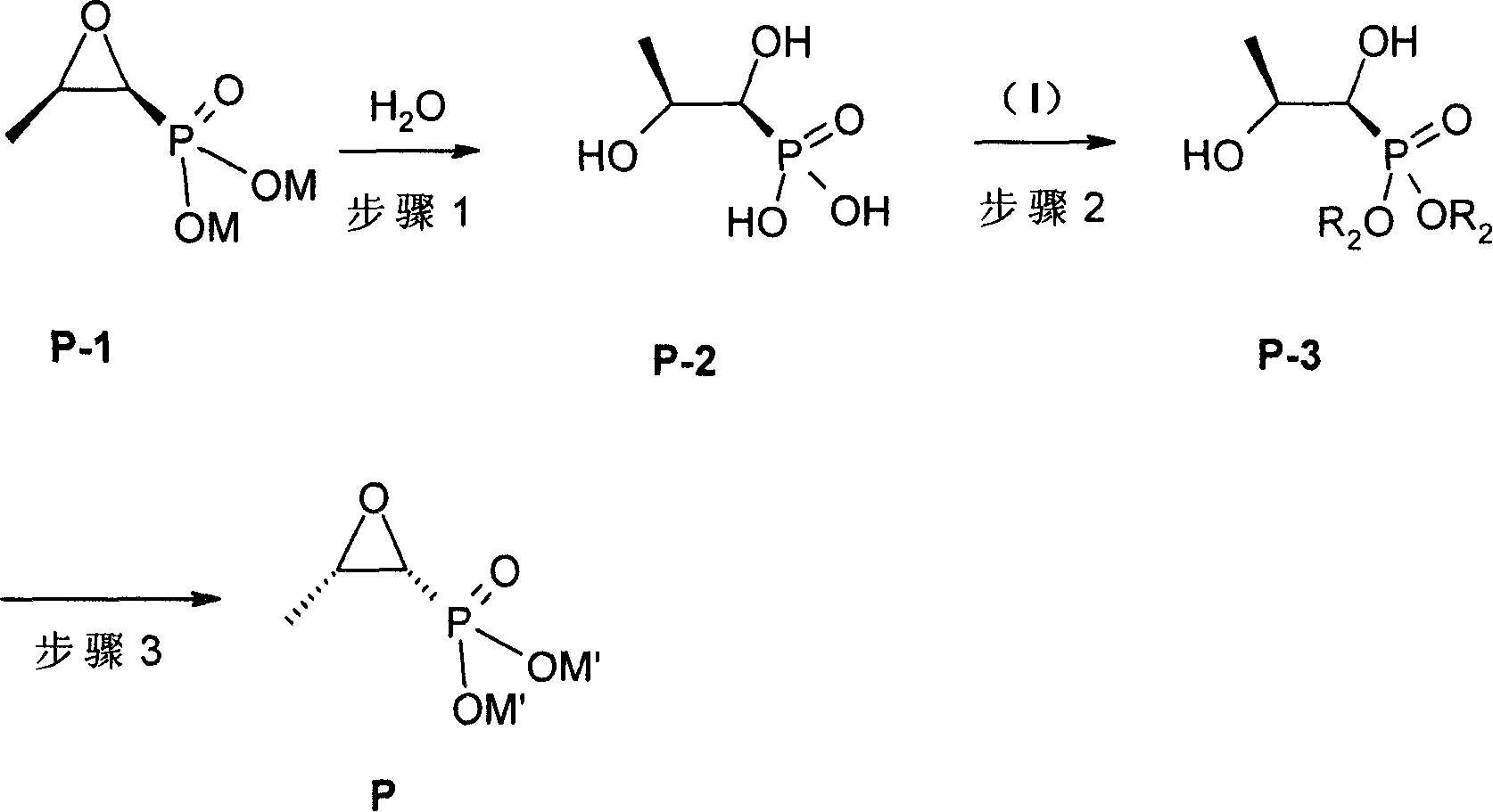
[0061] Preparation of levorotatory from (-) levorotatory α-methylbenzylamine salt ((+)-[1S,2R]-cis-glycidyl phosphate · (-) levorotatory α-phenylethylamine salt) Fosfomycin sodium ((-)-[1R,2S]-cis-glycidyl phosphate sodium)
[0062] Step 1: Preparation of [1S,2S]-dihydroxypropyl phosphate from (-)L-α-methylbenzylamine salt of D-fosfomycin
[0063](-) L-alpha-methylbenzylamine salt (25.9g, 0.10mol) of dexfosfomycin was dissolved in 80ml of water, under stirring at room temperature, 20ml of aqueous solution of sodium hydroxide (8.0g, 0.20mol) was added, separated, and water The phase was extracted with 40 ml of dichloromethane. Take the water phase, add 98% concentrated sulfuric acid (10 g, 0.10 mol), stir, and heat to reflux for 4 h. Concentrate under reduced pressure to remove most of the water, add 70ml of methanol and 30ml of ethanol, stir for 5min, let stand for 0.5h, filter, concentrate the filtrate, and dry in vacuo to obtain 15.5g of [1S,2S]-dihydroxypropylphosphoric a...
Embodiment 2
[0080] Preparation of Levofosfomycin Sodium ((-)-[1R,2S]-cis-Glycidyl Phosphate from Defosfomycin Sodium ((+)-[1S,2R]-cis-Glycidyl Phosphate) calcium phosphate)
[0081] Step 1: Preparation of [1S,2S]-dihydroxypropyl phosphate from dexfosfomycin sodium ((+)-[1S,2R]-cis-glycidyl phosphate sodium)
[0082] Defosfomycin sodium (24g, 0.13mol) was dissolved in 100ml of water, under stirring at room temperature, was added 70% perchloric acid aqueous solution (37.3g, 0.26mol), stirred, and heated to reflux for 4h. Concentrate under reduced pressure to remove most of the water, add 80ml of methanol, 50ml of isopropanol, stir for 5min, let stand for 0.5h, filter, concentrate the filtrate, and dry in vacuo to obtain 19.8g of [1S, 2S]-dihydroxypropyl phosphoric acid, the yield 96%.
[0083] Note: the NMR data is consistent with the data in Example 1.
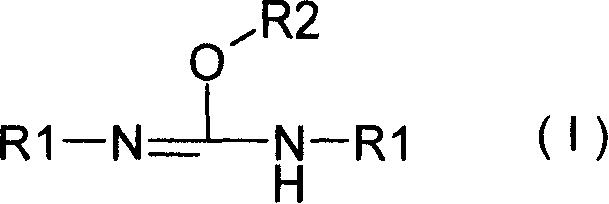
[0084] Step 2: Preparation of [1S,2S]-Dibenzyl Dihydroxypropyl Phosphate from [1S,2S]-Dihydroxypropyl Phosphate
[0085] N,N'-diisopr...
Embodiment 3
[0100] Preparation of Levofosfomycin Sodium ((-)-[1R,2S]-cis-Glycidyl Phosphate from Defosfomycin Calcium ((+)-[1S,2R]-cis-Glycidyl Calcium Phosphate) Sodium Phosphate)
[0101] Step 1: Preparation of [1S,2S]-dihydroxypropyl phosphate from the calcium salt of dexfosfomycin
[0102] Add the calcium salt of dexfosfomycin (17.6g, 0.10mol) in 70ml of water, under stirring at 40°C, add 5mol / L sulfuric acid aqueous solution (20ml) dropwise, after the addition is complete, let stand at 40°C for 4h, filter, and Heated to reflux for 4h. Concentrate under reduced pressure to remove most of the water, add 50ml of methanol, stir for 5min, let stand for 0.5h, filter, concentrate the filtrate, and dry in vacuo to obtain 14.9g of [1S,2S]-dihydroxypropylphosphoric acid with a yield of 96%.
[0103] Note: the NMR data is consistent with the data in Example 1.
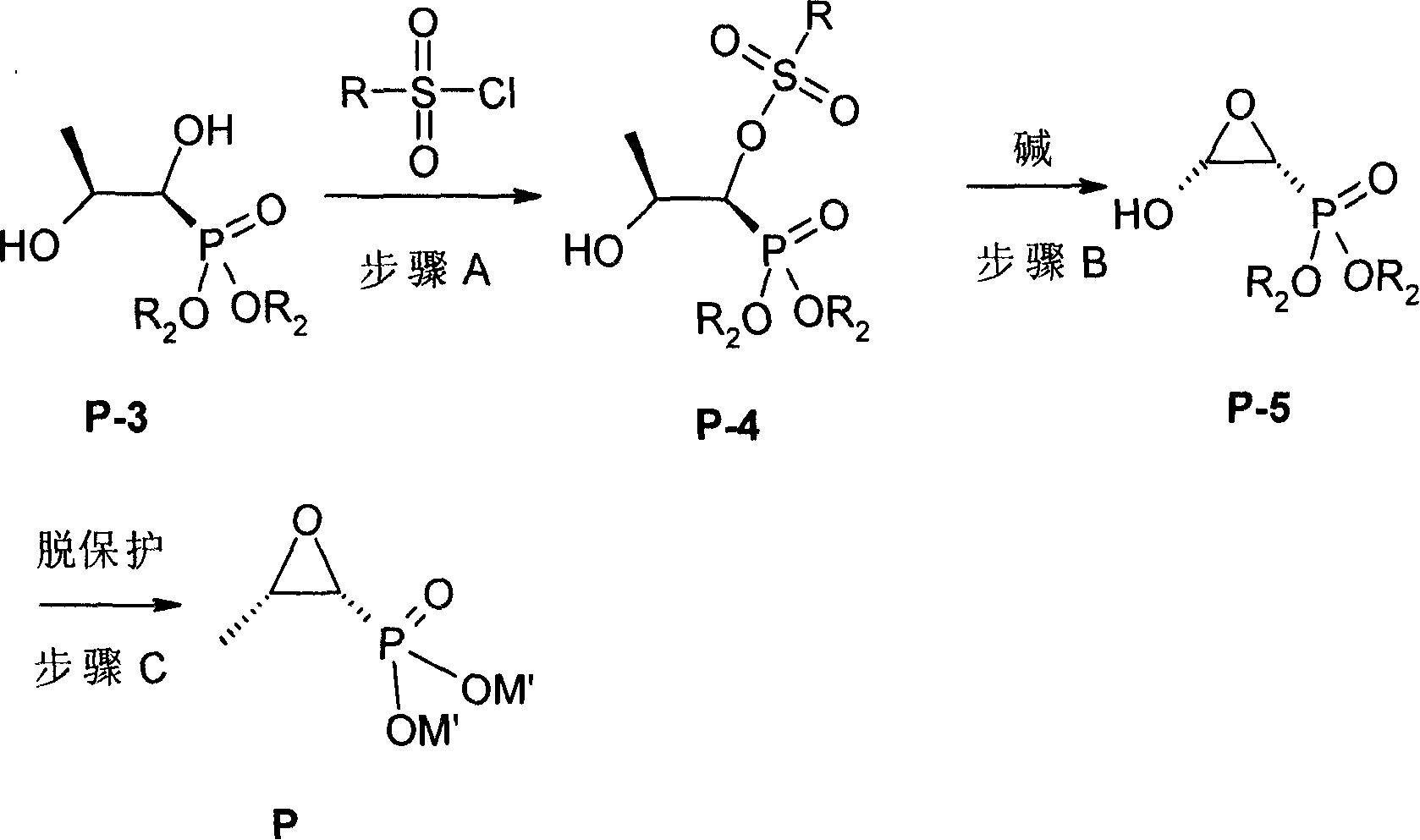
[0104] Step 2: Preparation of [1S,2S]-dihydroxypropylphosphate di-o-chlorobenzyl ester from [1S,2S]-dihydroxypropylphosphate
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com