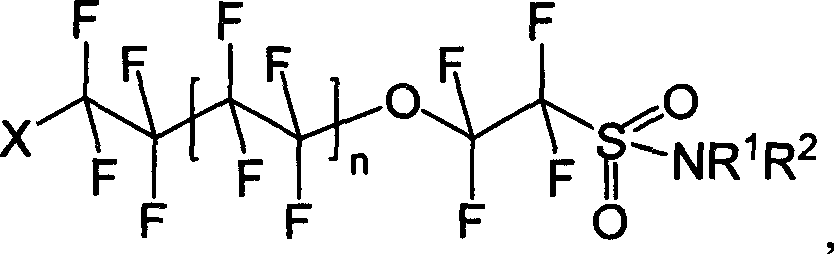
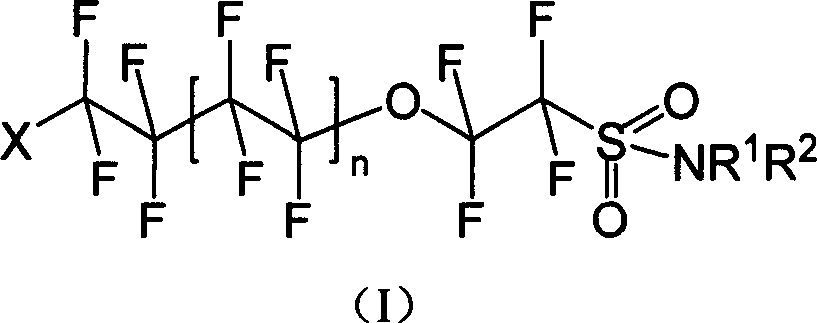
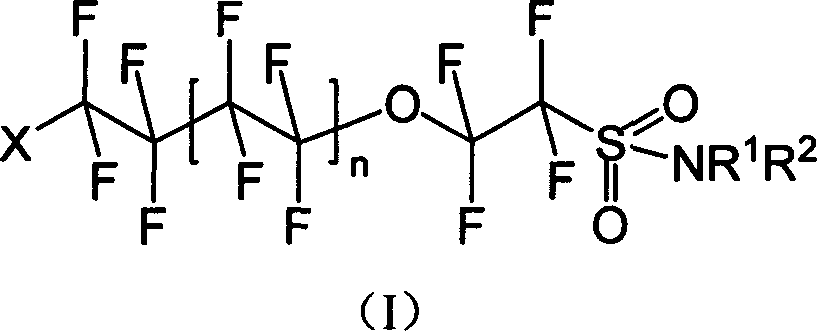
Oxo fluoroalkyl sulfamide compound and its preparing method and use
The technology of heterofluoroalkylsulfonamide and amine compounds is applied in the field of sanitation and agrochemical pesticides, which can solve the problems of large harm and achieve the effects of reasonable toxicity, good cockroach killing activity and good hygienic insecticidal activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of I-1: Dissolve 8.520g (20mmol) of (II) in ether, add 5.228g (60mmol) of morpholine, stir and react for 8h under dark conditions, wash with water and saturated NaCl aqueous solution successively, separate liquids, and diethyl ether The layer was dried with anhydrous sodium sulfate and spin-dried to obtain 4.592 g of white solid I-1 with a yield of 46.56%.
[0041] Mp (melting point): 64-66°C
[0042] 1 H NMR (nuclear magnetic spectrum) (CD 3 Cl) (ppm): 3.77 (4H, s), 3.56 (4H, t).
[0043] 19 F NMR (CD 3 Cl) (ppm) (fluorine nuclear magnetic spectrum): -65.11 (2F, s), -82.27 (2F, t), -85.68 (2F, d), -115.86 (2F, s).
Embodiment 2
[0045] Synthesis of I-2: Dissolve 8.520 g (20 mmol) of (II) in diethyl ether, add 5.109 g (60 mmol) of hexahydropyridine, stir and react for 8 h under dark conditions, wash with water and saturated NaCl aqueous solution successively, and separate the liquids. The ether layer was dried over anhydrous sodium sulfate, spin-dried and distilled under reduced pressure. 6.815 g (1-2 mmHg, 106-108° C.) of pale yellow liquid of I-2 was obtained with a yield of 69.38%.
[0046] 1 H NMR (CD 3 Cl) (ppm): 3.70 (2H, m), 3.33 (2H, m), 1.68 (6H, s).
[0047] 19 F NMR (CD 3 Cl) (ppm): -65.07 (2F, s), -82.37 (2F, t), -85.79 (2F, d), -116.27 (2F, s).
Embodiment 3
[0049] Synthesis of I-3: Dissolve 8.520g (20mmol) of (II) in diethyl ether, add 4.38g (60mmol) of n-butylamine, stir and react for 8h under dark conditions, wash with water and saturated NaCl aqueous solution successively, and separate the liquids. The ether layer was dried over anhydrous sodium sulfate, spin-dried and distilled under reduced pressure. A pale yellow liquid of I-3 was obtained with a yield of 72.1%.
[0050] 1 H NMR (DMSO) (ppm): 3.15 (2H, t), 1.47 (2H, m), 1.31 (2H, m), 0.86 (3H, t)
[0051] 19 F NMR (DMSO) (ppm): -65.08 (2F, s), -82.27 (2F, t), -85.59 (2F, d), -116.30 (2F, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com