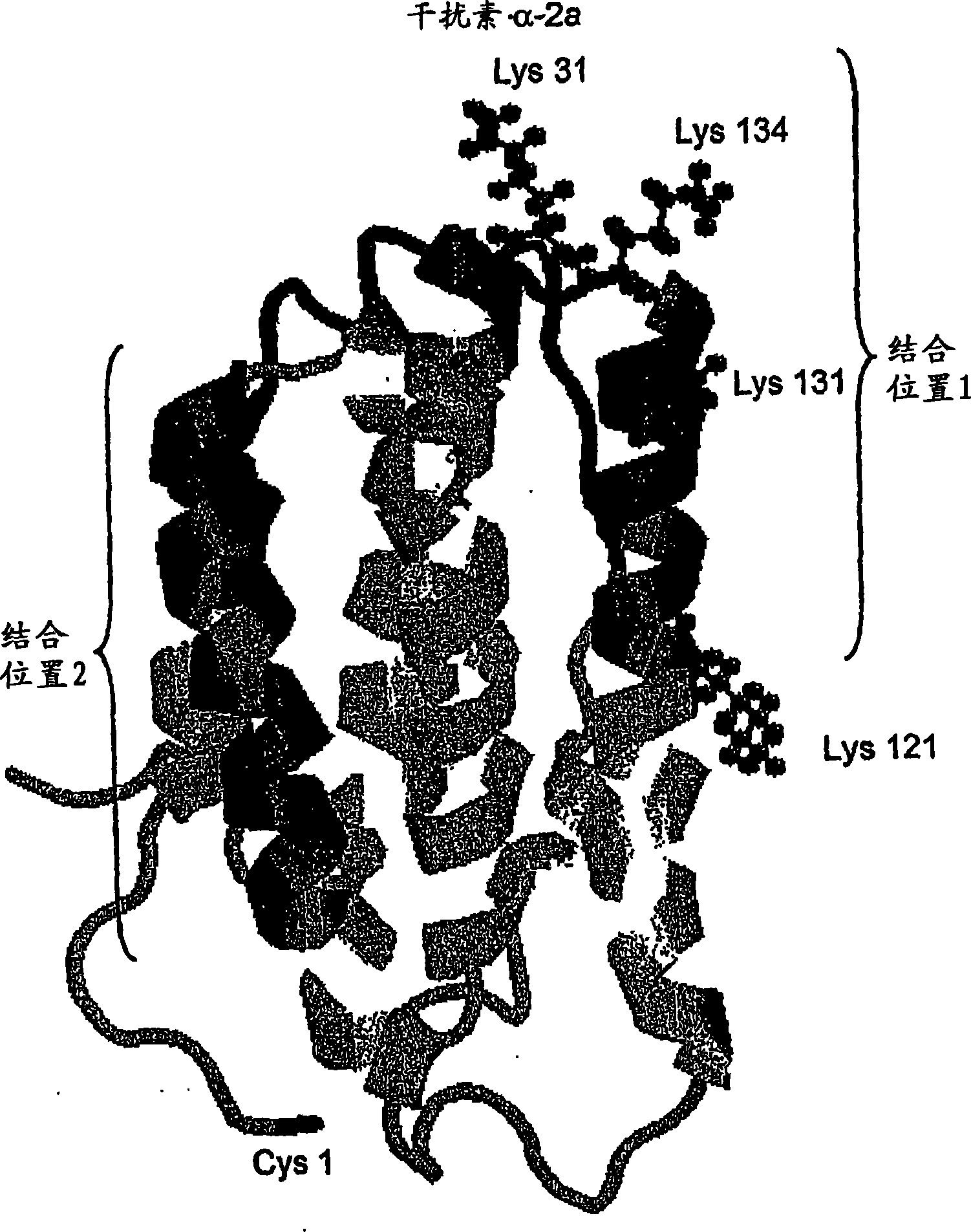
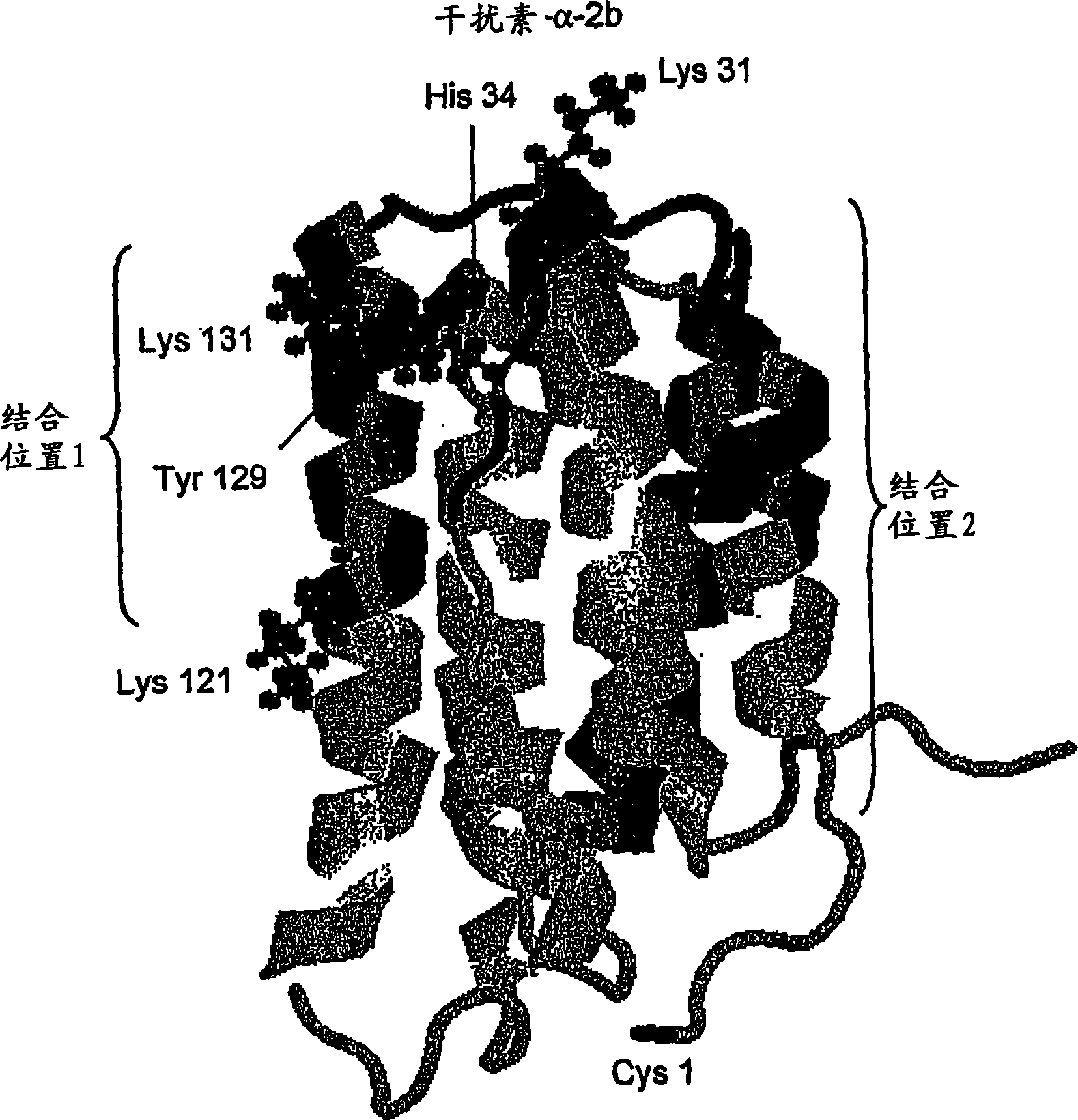
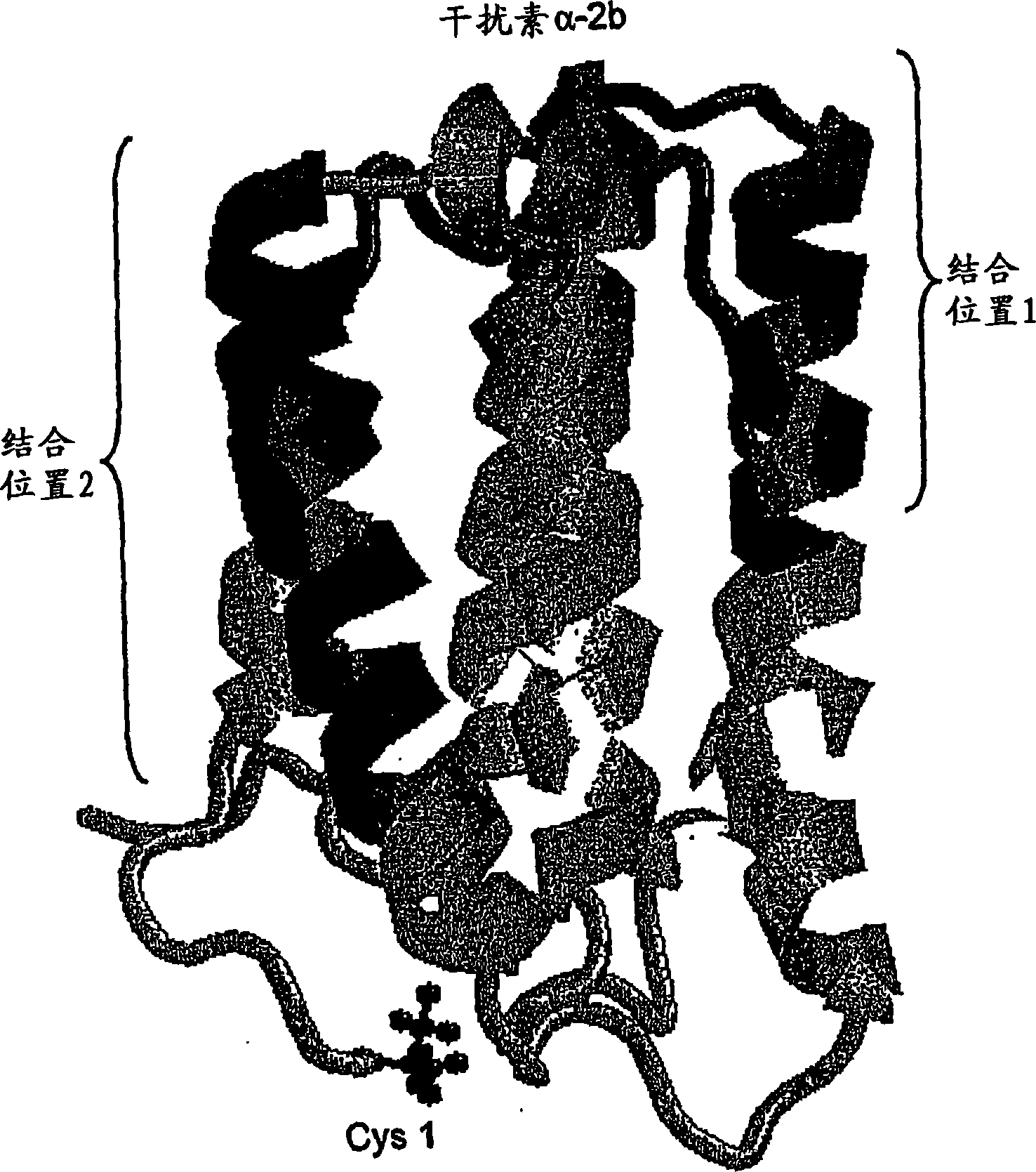
Polymer conjugates of cytokines, chemokines, growth factors, polypeptide hormones and antagonists thereof with preserved receptor-binding activity
A technology of cytokines and growth factors, applied in the fields of protein biochemistry, pharmacy and medicine, which can solve problems such as large size and space obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1: PEG-interferon-alpha conjugates
[0171] Interferon-alpha is a commercially important medical protein with a global market of more than $2 billion in 2001, mainly used to treat patients infected with hepatitis C virus ("HCV"). In the United States, 3 to 4 million people are chronically infected with hepatitis C virus, and approximately 10,000 people die each year from HCV-related causes (Chander, G., et al., (2002) Hepatology 36:5135-5144). In an effort to improve the usefulness of interferon-alpha, the two main companies responsible for the development and marketing of interferon-alpha (Schering-Plough and F. Hoffmann-La Roche AG) ) has developed a conjugate of interferon-alpha with monomethoxypoly(ethylene glycol) or "mPEG" and has released this product commercially. In each case, mPEG was attached to each molecule of interferon-α at only one point of attachment. In each case, the product contained a mixture of positional isomers with significantly reduce...
Embodiment 2
[0176] Example 2: PEG-Interleukin-2 Conjugates
[0177] Interleukin-2 ("IL-2") is a cytokine that exhibits immunomodulatory activity against certain cancers, including renal cell carcinoma and malignant melanoma. However, clinical efficacy is poor, with the result that only a small fraction of patients experience partial or complete responses (Weinreich, D.M., et al., (2002) J Immunother 25:185-187). The short half-life of IL-2 in the bloodstream makes it slow to induce remission in cancer patients. Attempts to make IL-2 more useful by random pegylation of lysine residues have not been ideal (Chen, S.A., et al. (2000) J Pharmacol Exp Ther 293:248-259). Attempts to attach PEG selectively to glycosylation sites of IL-2 (Goodson, R.J., et al., supra), or to IL-2 containing cysteine (between residues 1 and 20) Attempts at non-essential cysteine (Cys125) or mutants thereof (Katre, N., et al., U.S. Patent No. 5,206,344) have not resulted in a clinically useful product.
[017...
Embodiment 3
[0180] Example 3: Synthesis and analysis of N-terminal PEGylated EGF and IGF-1
[0181] respectively according to Figure 5 and 7 In the molecular model (which shows EGF and IGF-1 as RN growth factors), epidermal growth factor ("EGF"; SEQ ID NO: 7) and insulin-like growth factor-1 ("IGF-1"; SEQ ID NO :9) to carry out N-terminal PEGylation. 5-kDa PEG-propionaldehyde (NOF Corporation, Tokyo) was dissolved in 1 mM HCl so that the final concentration became 15 mg / ml to prepare a 3 mM solution of 5-kDa PEG-aldehyde. Borane-pyridine was prepared by diluting 35 microliters (mcL) of 8M borane-pyridine (Aldrich) in 0.3 mL of acetonitrile plus 0.15 mL of water to a final concentration of 0.58M. A buffer (pH 6.3) containing 0.2M each of sodium phosphate and sodium acetate was prepared and filtered through a 0.1 micron pore sterile filter. Recombinant human EFG from Invitagen (Casbain, CA) was dissolved in water at a concentration of 1 mg / ml. To 0.6 ml of this solution were added 70 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com