Organic polyamine, its synthesizing process and use
The technology of an organic polyamine and a synthesis method, which is applied in the synthesis field of the organic polyamine, can solve the problems of few types of effective functional groups and limited application range, and achieves the effects of wide application range, many types and strong coordination ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
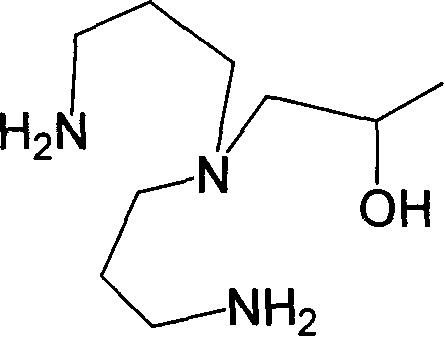
[0028] Example 1. A kind of organic polyamine, its structural formula is as follows:
[0029]
[0030] Its chemical name is: 1-[di(3-aminopropyl)amino]-2-propanol;
[0031] Molecular formula is C 9 h 23 N 3 O, the molecular weight is 189.
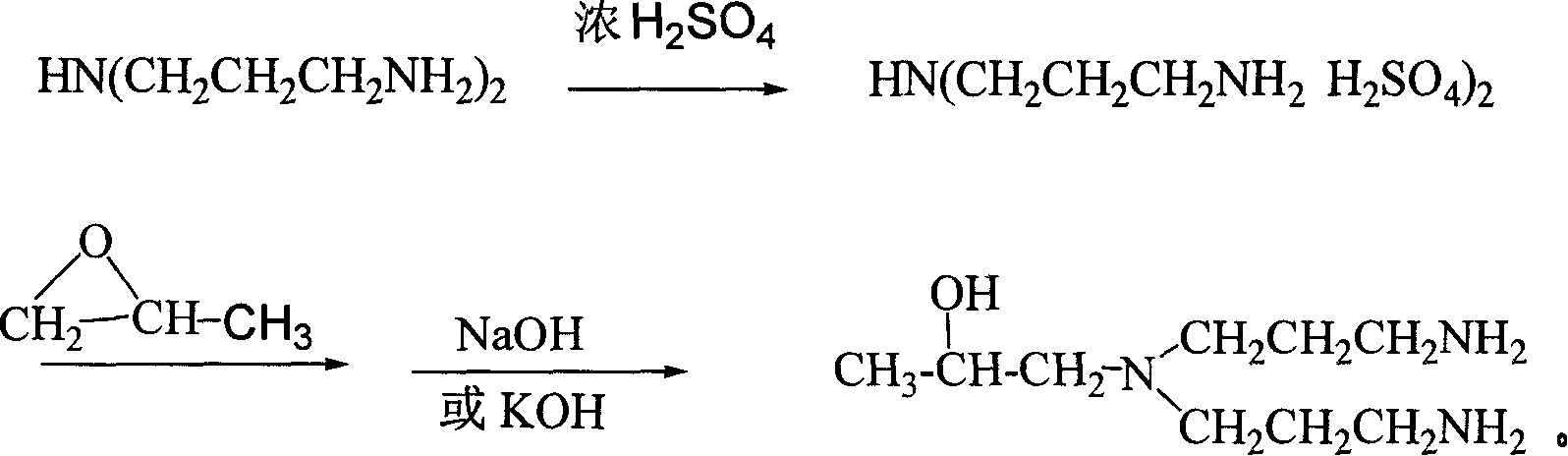
[0032] The following method is used to synthesize, on the basis of the protection of the primary amino group in concentrated sulfuric acid, the target product 1-[two (3- Aminopropyl) amino]-2-propanol, the synthetic reaction equation is:
[0033]
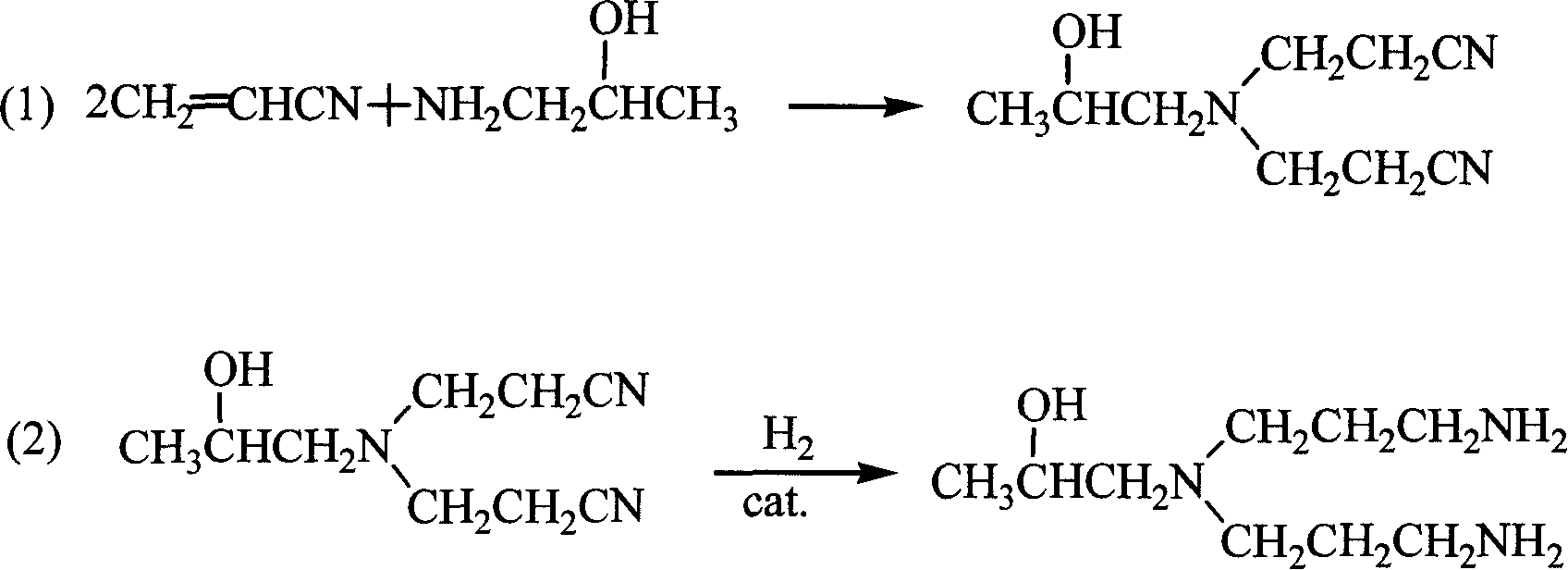
[0034] The 1-[bis(3-aminopropyl)amino]-2-propanol of this embodiment can be used as a catalyst for the preparation reaction of benzyl nitrile. In the preparation reaction of benzyl nitrile, the ratio of the amount of benzyl chloride, sodium cyanide and 1-[bis(3-aminopropyl)amino]-2-propanol is 1:1.08:0.003.
Embodiment 2
[0035] Example 2. A kind of organic polyamine, its structural formula is as follows:
[0036]
[0037] Its chemical name is: 1-[di(3-aminopropyl)amino]-2-propanol;
[0038] Molecular formula is C 9 h 23 N 3 O, the molecular weight is 189.
[0039]Synthesize using the following method, pour bis(3-aminopropyl)amine into the container, stir mechanically, then add water, then add 70% concentrated sulfuric acid dropwise, cool to below 30°C and let it stand for several hours. Add 1,2-propylene oxide to the above system at a rate of 1 mL / h to continue the reaction. Under cooling conditions, adjust the pH to 11 with KOH, filter out the solid inorganic salt, and distill under reduced pressure to remove unreacted substances. Collect The fraction at 175-176°C / 10mmHg yields the target compound 1-[bis(3-aminopropyl)amino]-2-propanol. The mass ratio of bis(3-aminopropyl)amine, water, concentrated sulfuric acid and 1,2-propylene oxide is: 1:0.5:0.8:0.2.
[0040] The 1-[bis(3-aminop...
Embodiment 3
[0041] Example 3. A kind of organic polyamine, its structural formula is as follows:
[0042]
[0043] Its chemical name is: 1-[di(3-aminopropyl)amino]-2-propanol;
[0044] Molecular formula is C 9 h 23 N 3 O, the molecular weight is 189.
[0045] Synthesize using the following method, pour bis(3-aminopropyl)amine into the container, stir mechanically, then add water, then add 98% concentrated sulfuric acid dropwise, cool to below 30°C and let it stand for several hours, under stirring condition Add 1,2-propylene oxide to the above system at a rate of 10 mL / h to continue the reaction. Under cooling conditions, adjust the pH to 15 with NaOH, filter out the solid inorganic salts, and distill under reduced pressure to remove unreacted substances. Collect The fraction at 175-176°C / 10mmHg yields the target compound 1-[bis(3-aminopropyl)amino]-2-propanol. The mass ratio of bis(3-aminopropyl)amine, water, concentrated sulfuric acid and 1,2-propylene oxide is: 1:~0.8:1.1:0.4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com