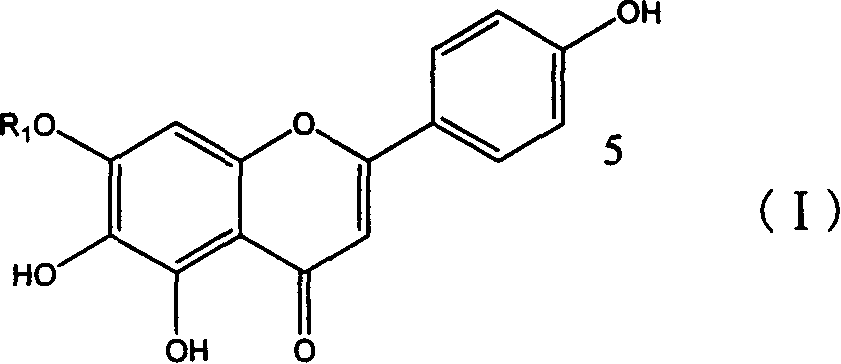
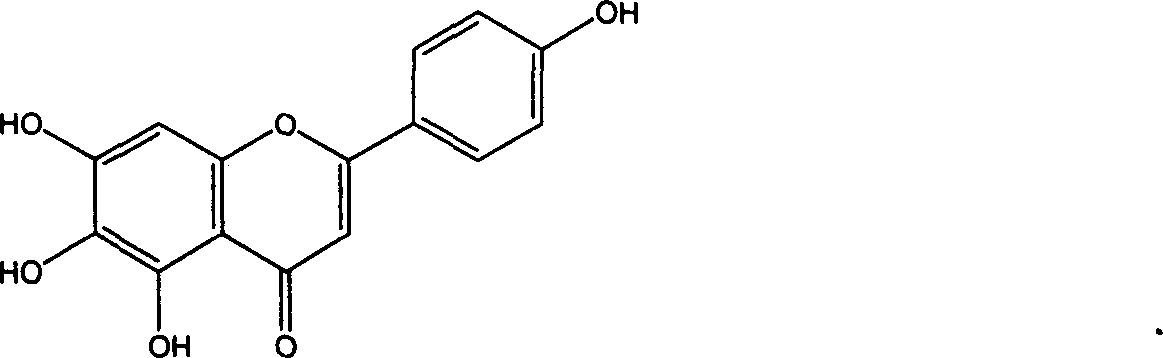
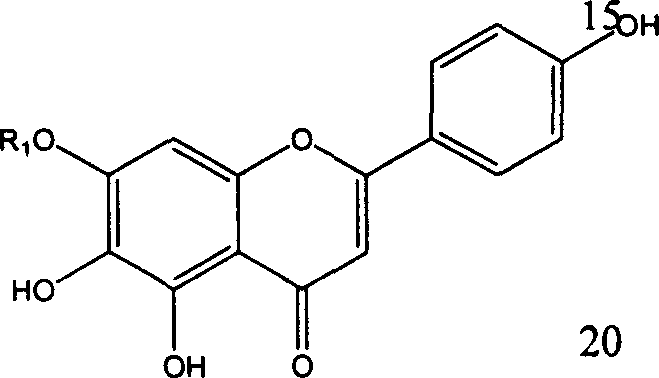
Application of erigeron breviscapus and Scutellarin in preparing medication anti tumor
A technology of scutellarin and scutellarin, applied in the field of pharmaceutical use of flavonoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Experiment of the inhibitory effect of scutellarin and scutellarin on the growth of tumor cells in vitro
[0029] Drugs to be tested: scutellarin and scutellarin (existing commercial products) were dissolved in dimethyl sulfoxide (DMSO), and used in a 1 / 1000 dilution during actual testing.
[0030]Positive control drug: 5-fluorouracil, dissolved in DMSO, diluted 1 / 1000 for actual testing.
[0031] Method and Results:
[0032] The effects of scutellarin and scutellarin on human lung cancer cell line A549, human leukemia cell line HL60, human gastric cancer cell line MKN28, human colorectal cancer cell line HCT116, human liver cancer cell line HepG2, human esophageal cancer The proliferation inhibition of cell line TE-1 and human breast cancer cell line MCF-7 was measured to measure the IC50 concentration, as well as the IC50 concentration of the positive control drug 5-fluorouracil. At the same time, normal human fibroblasts were used as a control.
[0033] ...
Embodiment 2
[0037] Example 2 Animal Experiment of the Inhibitory Effect of Scutellarin on the Growth of Transplanted Tumor Cells in Mice
[0038] About 20 grams of C57 purebred mice 10 as a group, divided into a control group, a group of high and low dosage groups, a total of three groups. Experiments were carried out to determine the inhibitory effect of scutellarin on the growth of transplanted tumor cells in mice.
[0039] 1×105 B16 melanoma cells were planted subcutaneously in each mouse. On the second day after tumor cell transplantation, 5 mg / kg was used as the low dosage group, and 15 mg / kg was used as the high dosage group, and scutellarin dissolved in tetraethylene glycol was injected into the abdominal cavity of the animals. Inject once every other day, and stop administration after 15 consecutive days of administration. On the 28th day after the start of administration, the mice were sacrificed, the tumor mass was peeled off, and the tumor growth inhibition rate was calculate...
Embodiment 3
[0042] Example 3 Animal experiment of scutellarin on the growth inhibitory effect of transplanted tumor cells in mice
[0043] About 20 grams of C57 purebred mice 10 as a group, divided into a control group, a group of high and low dosage groups, a total of three groups. Experiments were conducted to determine the inhibitory effect of scutellarin on the growth of transplanted tumor cells in mice.
[0044] 1 × 10 cells were implanted subcutaneously in each mouse 5 B16 melanoma cells. On the second day after tumor cell transplantation, 10 mg / kg was used as the low dose group, and 30 mg / kg was used as the high dose group, and scutellarin dissolved in tetraethylene glycol was injected into the abdominal cavity of the animal. Inject once every other day, and stop administration after 15 consecutive days of administration. On the 28th day after the start of administration, the mice were sacrificed, the tumor mass was peeled off, and the tumor growth inhibition rate was calculated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com