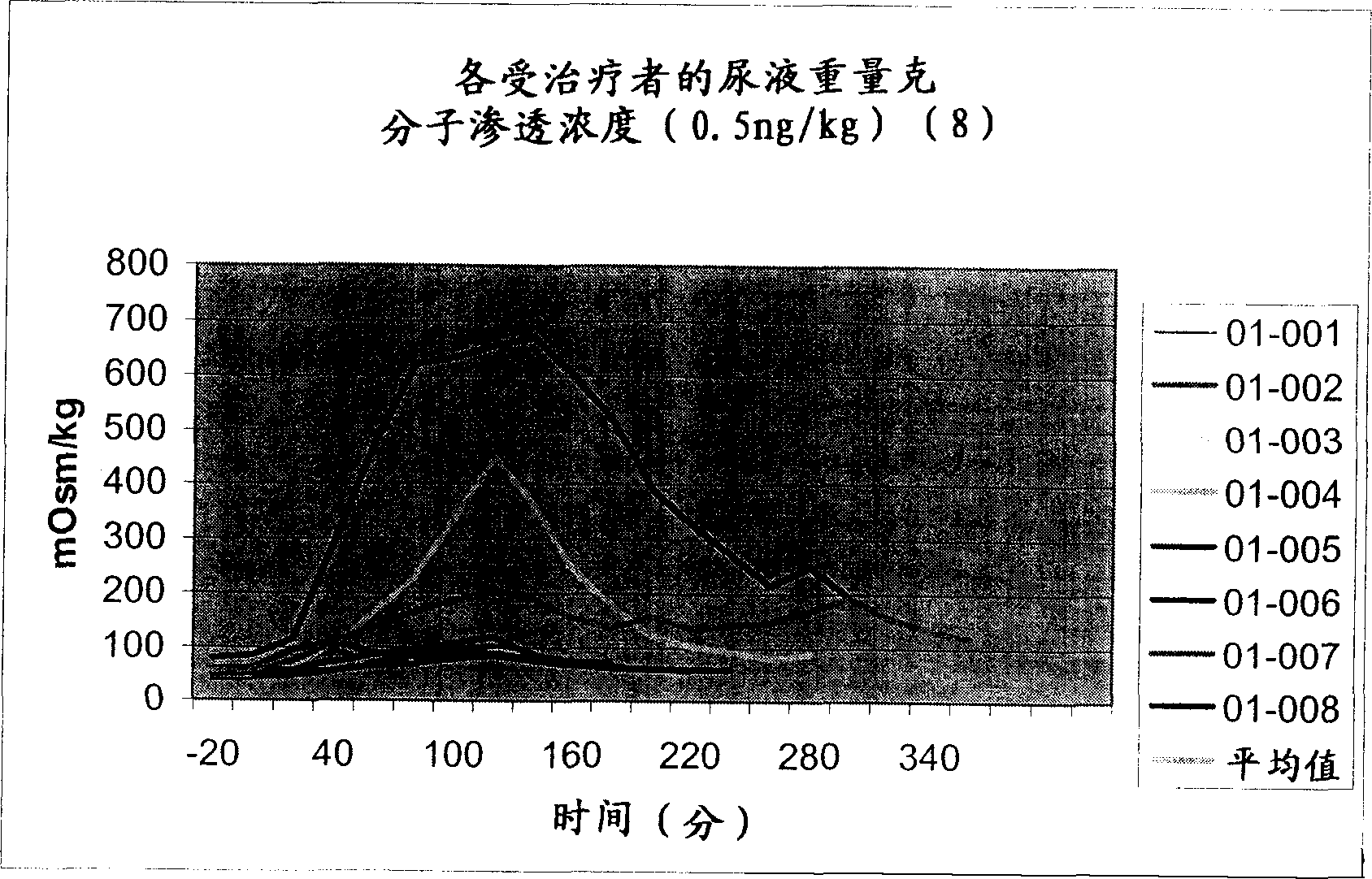
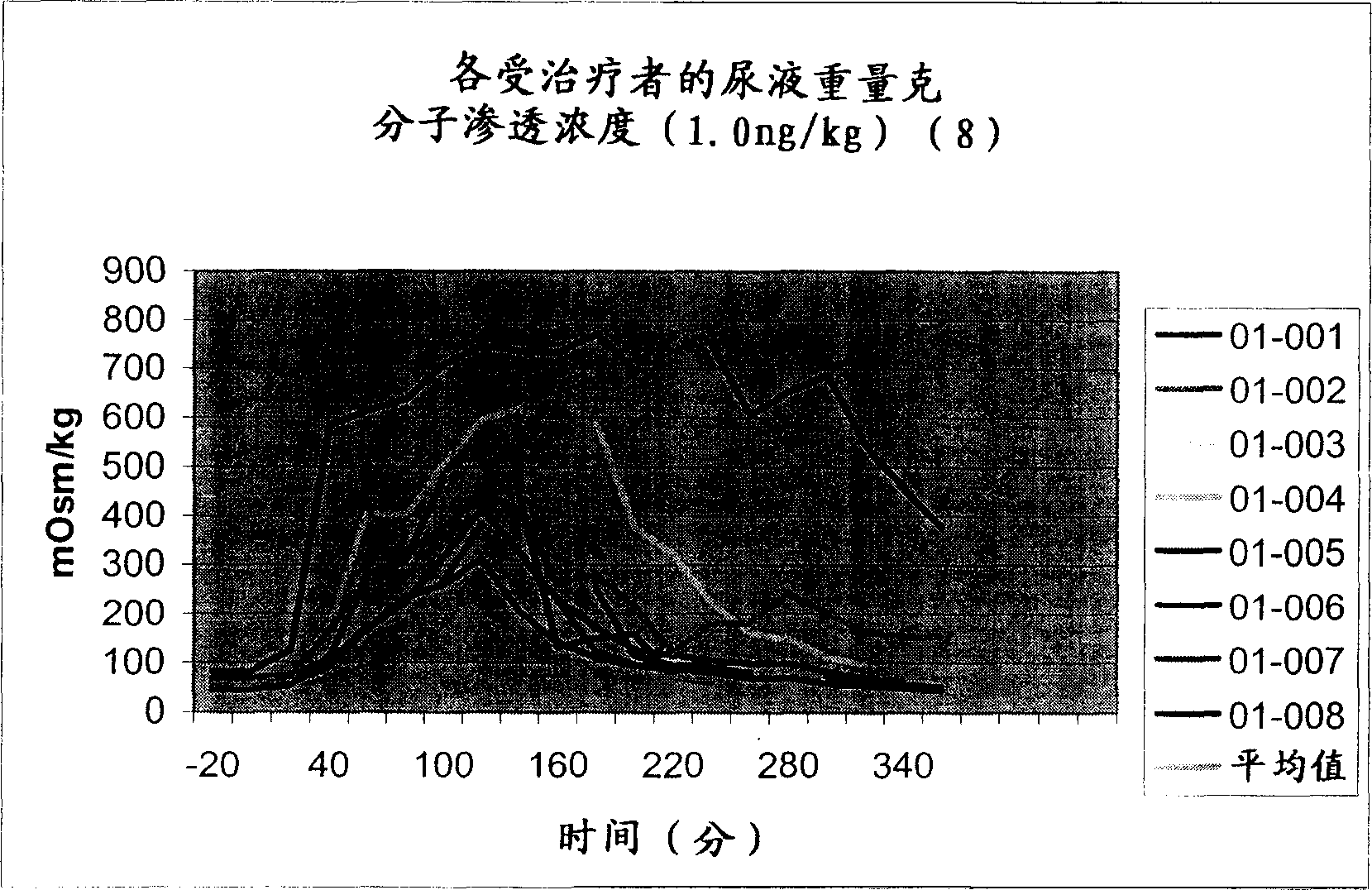
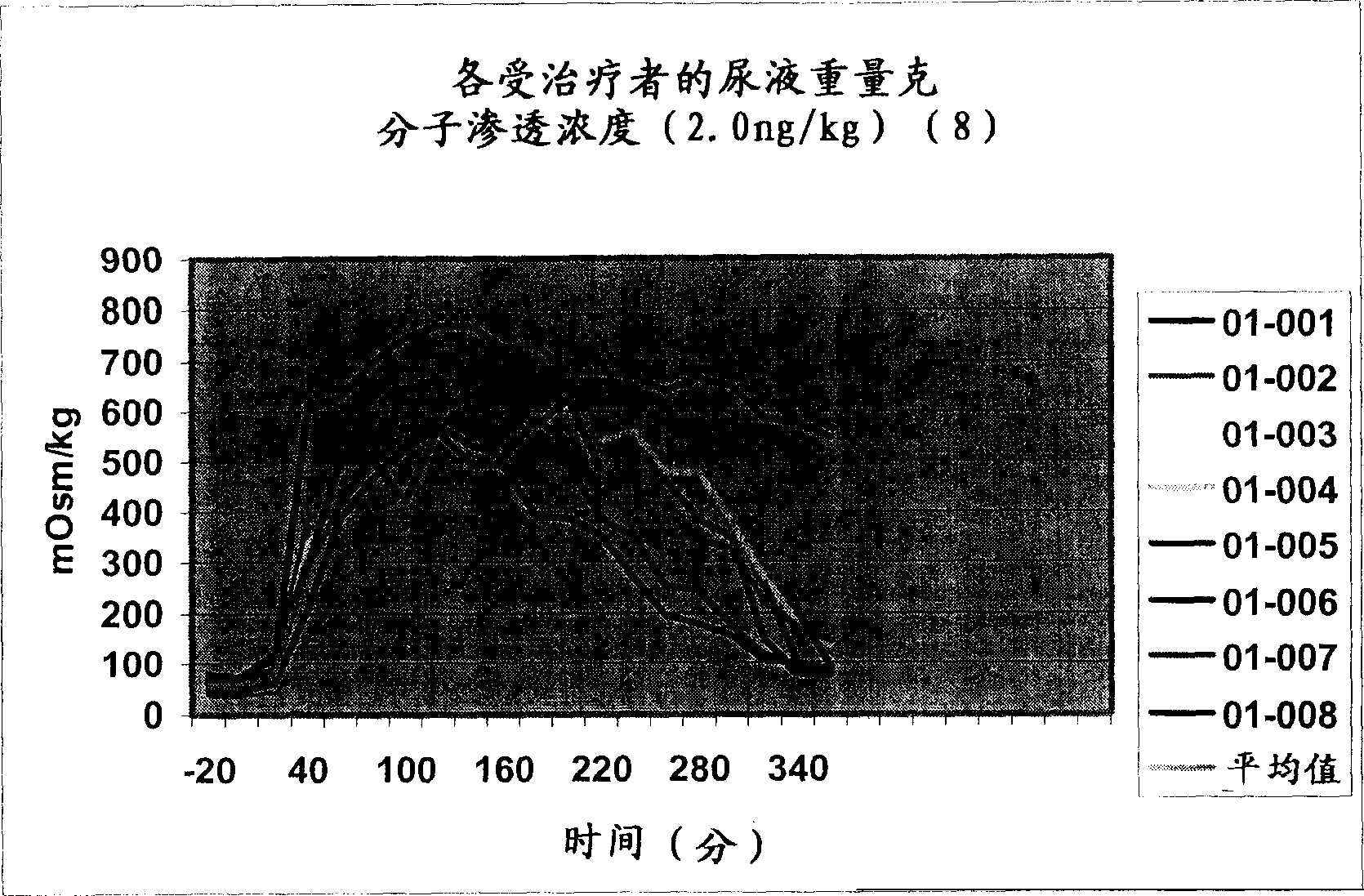
Pharmaceutical compositions including low dosages of desmopressin
A technology for desmopressin and composition, which can be applied in the directions of drug combination, drug delivery, medical preparations containing active ingredients, etc., and can solve problems such as adverse side effects, fluid restriction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1 Orodispersible dosage form of 200 μg desmopressin
[0118] Spray-dried fish gelatin (4 g) and mannitol (3 g) were added to a glass beaker. Purified water (93 g) was then added and the solution was stirred using a magnetic follower. Check the pH and adjust to 4.8 with citric acid if necessary. A Gilson pipette can then be used to deliver 500 mg of this solution into a series of pre-formed individual blister wells having a well diameter of approximately 16 mm. The blister laminate may comprise PVdC coated PVC. The dosing unit was then frozen in a freezing tunnel at -110°C for a residence time of 3.2 minutes, and then placed in an upright freezer at -25°C (±5°C) for more than 1.5 hours. The units were then lyophilized overnight at a pressure of 0.5 mbar with an initial storage temperature of 10°C raised to +20°C. The unit can be checked for moisture by drying the trace and passing a pressurized moisture check before removing the load.
[0119] In this manne...
Embodiment 2
[0125] Example 2 Orodispersible dosage form of 400 μg desmopressin
[0126] The procedure of Example 1 herein was followed except that the amount of desmopressin per unit dosage form was 400 μg.
Embodiment 3
[0127] Example 3 Orodispersible dosage form of 800 μg desmopressin
[0128] The procedure of Example 1 herein was followed except that the amount of desmopressin per unit dosage form was 800 [mu]g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com