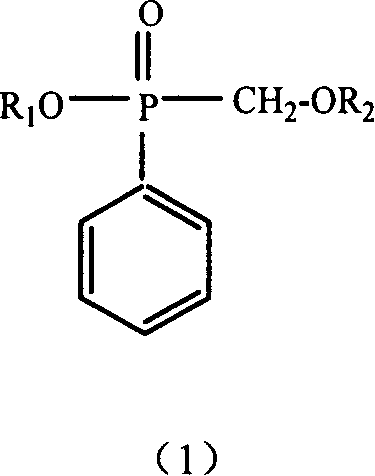
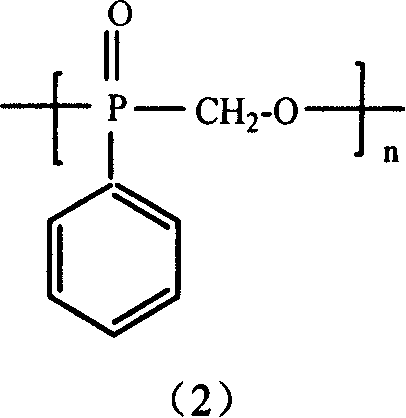
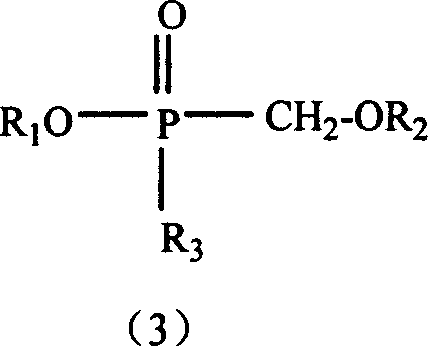Method for the production of hydroxymethyl alkyl phosphinic acids and its ester thereof
A technology for hydroxymethyl hydrocarbyl phosphinic acid and hydrocarbyl phosphinic acid ethyl ester is applied in the field of preparation of hydroxymethyl hydrocarbyl phosphinic acid and its ester compound, and achieves the effects of rich and diverse raw material sources and green synthesis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Synthesis of ethyl phenylphosphonite: the phenylphosphine dichloride of 44.75g (0.25 mole) is dripped in the 250 milliliter four-neck flask that 46g (1 mole) ethanol is housed under stirring , control the dropwise addition temperature at 5-10°C, complete the addition in 30 minutes, then raise the temperature to 30°C for 2 hours, and pass the generated hydrogen chloride gas into another flask, absorb it with water to form hydrochloric acid. Raise the temperature to 75°C, distill under reduced pressure, distill unreacted ethanol and ethyl chloride, and the residual liquid is ethyl phenylphosphinate, which is a colorless, slightly viscous liquid, which can be used directly without purification. Next reaction.
Embodiment 2
[0017] Embodiment 2: Synthesis of hydroxymethylphenylphosphinic acid: get 50g of ethyl phenylphosphinate synthesized in Example 1, put it into a 250ml four-neck flask, heat to 80°C, and add 15g in batches After the addition of paraformaldehyde, the temperature was raised to 110°C for 1 hour to complete the reaction. Then add 100 ml of 10% dilute hydrochloric acid and keep the temperature at 70° C. for 1 hour. Part of the water, hydrogen chloride, ethyl chloride, etc. were evaporated under reduced pressure. After cooling down, a white solid could be obtained by filtration. The resulting solid was recrystallized in water, filtered, and dried to obtain 43 g of the target product, hydroxymethylphenylphosphinic acid, with a melting point of 138° C. and an acid value of 232.1 mg NaOH / g (theoretical value 232.52 mg NaOH / g).
Embodiment 3
[0018] The synthesis of embodiment 3 hydroxymethylphenylphosphinic acid ethylene glycol esters: add ethylene glycol 134g, hydroxymethylphenylphosphinic acid 43g in a 250 milliliters of there-necked flasks equipped with oil bath, stirring and rectifying column , start stirring, heat up to 140°C, reflux occurs in the rectification column, take out part of the water generated by the reaction, control the reflux ratio, heat up to 160-180°C, and a large amount of water is separated through the rectification column. After 2 hours of reaction time, the reaction was stopped, and the temperature was lowered to obtain 156 grams of a solution containing ethylene glycol and ethylene glycol hydroxymethylphenyl phosphinate. The solution can be directly used in the preparation of flame-retardant polyester.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com