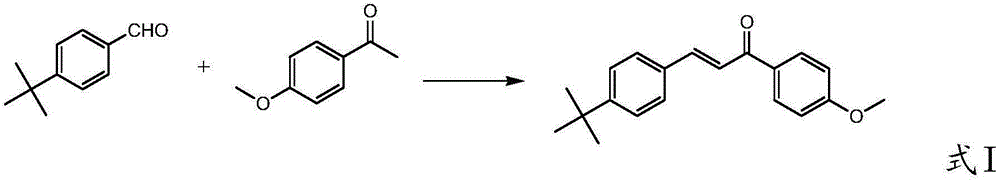
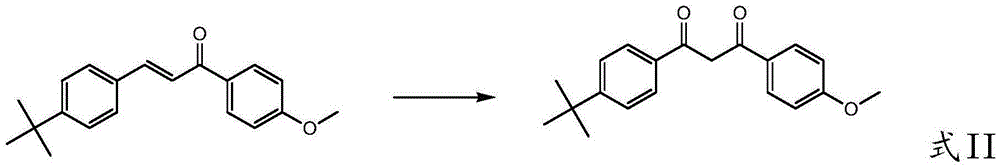
Method for preparing avobenzone
A technology for avobenzone and benzaldehyde, which is applied in the field of preparing avobenzone, and can solve the problems of high cost, no price advantage, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] The method for preparing avobenzone provided by the present invention and the avobenzone prepared by the method have the following beneficial effects:
[0100] (1) The solvent used in the present invention and basic catalyst are environment-friendly, catalyze p-tert-butylbenzaldehyde and p-methoxyacetophenone condensation to prepare 3-((4-tert-butyl)phenyl)-1-(4 -Methoxyphenyl)-2-propen-1-one, the catalyst is cheap and easy to get, the reaction conditions are mild, the reaction is homogeneous at first, and then the product is gradually precipitated, and the product can be separated by low-temperature crystallization and filtration, and the product yield is High, high purity, and the solvent used can be recycled, the synthesis process is simple and environmentally friendly;
[0101] (2) Under the action of a catalyst with a cheap and easy-to-get oxidant, the oxidation of 3-((4-tert-butyl)phenyl)-1-(4-methoxyphenyl)-2-propene-1-one can Avobenzone can be directly prepared...
Embodiment 1
[0106] (1) Prepare a solution of 150.2g (1.0mol) p-methoxyacetophenone and 200g (6.25mol) methanol for use in a dry 2000mL container with stirrer, thermometer, constant pressure dropping funnel and condenser In the four-neck flask, add 800g (25.0mol) of methanol, start stirring, at 25°C, slowly add 3.1g (0.078mol) of solid sodium hydroxide, stir and dissolve, then add 162.2g (1.0mol) of p-tert-butylbenzaldehyde , control the reaction temperature at 25°C, and slowly add the methanol solution of p-methoxyacetophenone dropwise within 2.5 hours. During the dropwise addition, crystals are gradually precipitated, and a large amount of crystals are precipitated in the later stage of the dropwise addition. After the dropwise addition, continue the insulation reaction for 1.5h;
[0107] (2) Use 4.77g (0.08mol) acetic acid to adjust pH=6.5~7, cool to 0~5°C, filter, wash the filter cake with 100g (3.12mol) cold methanol (5°C), and filter the cake at -0.1MPa , and dried at 70°C for 10h t...
Embodiment 2
[0111] (1) 150.2g (1.0mol) of p-methoxyacetophenone and 160g (5mol) of methanol were prepared into a solution for use. In a dry 2000mL four-neck flask equipped with a stirrer, thermometer, constant pressure dropping funnel and condenser tube, add 640g (20.0mol) methanol, start stirring, and slowly add 2g (0.05mol) of solid hydrogen at 20°C Add 81.1 g (0.5 mol) of p-tert-butylbenzaldehyde after stirring and dissolving sodium oxide. Control the reaction temperature at 45-50°C, slowly add the methanol solution of p-methoxyacetophenone dropwise within 3 hours, after the dropwise addition, continue the heat preservation reaction for 1.2 hours.
[0112] (2) Use acetic acid to adjust pH=5~5.5, cool to 2°C, filter, wash the filter cake with 160g (5mol) of cold methanol (2°C), dry the filter cake at -0.1MPa, 75°C for 8h, and obtain the condensate 3-((4-tert-butyl)phenyl)-1-(4-methoxyphenyl)-2-propen-1-one 241.4g, melting point 114.0~115.9℃, HPLC purity 99.21%, yield 82.0 % (as p-tert...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com