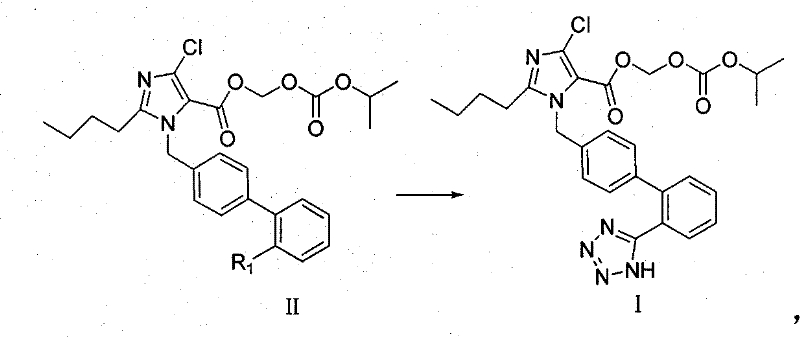
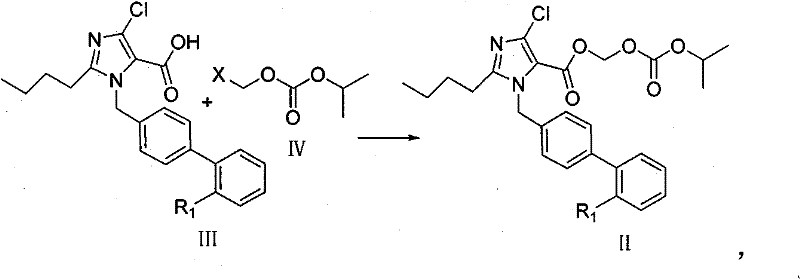
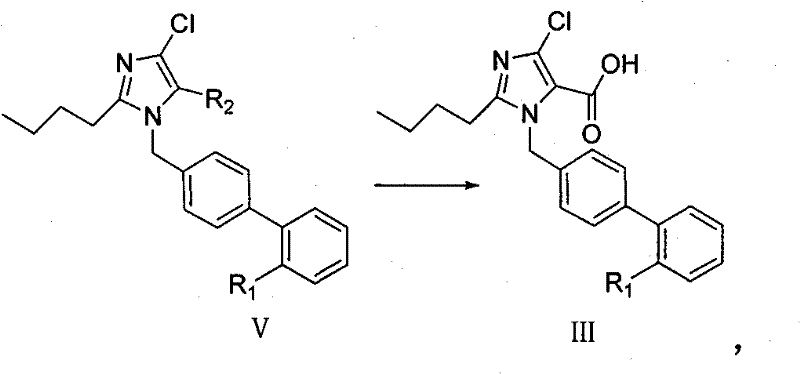
Imidazole-5-carboxylic acid derivant and method of preparing the same
A compound and azide technology, applied in the field of drug synthesis, can solve the problem of high polarity of the molecular structure of EXP3174, and achieve the effects of rich and diverse raw material sources, low production cost and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 2-Butyl-4-chloro-1-[2'-(1-trityl-1H-tetrazol-5-yl)-1,1'-biphenyl-methyl]-5-hydroxymethyl Base-1H-imidazole
[0041] 37.7 g of 2-butyl-4-chloro-5-hydroxymethyl-1H-imidazole, 4-bromomethyl-2'-(1-trityl-1H-tetrazol-5-yl)-linked Benzene 111.5g, K 2 CO 3 56g and 800mL of anhydrous DMF were mixed, and the reaction was stirred at 90°C for 6h. Cool, add 1000g of ice water, and continue stirring for 1h. Extracted with ethyl acetate, washed the organic phase with water, washed with saturated NaCl solution, anhydrous NaCl 2 SO 4 dry. Concentrate under reduced pressure to a small volume, cool, place, and precipitate a solid, filter with suction, wash with a small amount of ethyl acetate, and dry to obtain the title 2-butyl-4-chloro-1-[2'-(1-trityl -1H-tetrazol-5-yl)-1,1'-biphenyl-methyl]-5-hydroxymethyl-1H-imidazole 113.0 g, yield 89.5%.
[0042] 1 H-NMR (CDCl 3 , 400MHz): δ0.80(t, 3H), δ1.25(m, 2H), δ1.49(m, 2H), δ2.56(t, 2H), δ4.73(s, 2H), δ5 .58(s, 2H), δ6.94-7.54(m, ...
Embodiment 2
[0044] 2-Butyl-4-chloro-1-[2'-(1-trityl-1H-tetrazol-5-yl)-1,1'-biphenyl-methyl]-imidazole-5- carboxylic acid
[0045] 2-Butyl-4-chloro-1-[2'-(1-trityl-1H-tetrazol-5-yl)-1,1'-biphenyl-methyl]-5-hydroxy Dissolve 6.60g of methylimidazole in 20mL of water, cool to below 0°C, add dropwise 150mL of 1% KMnO 4 Aqueous solution, after dripping, was stirred and reacted at 50°C for 16h. Suction filtration, add 50mlL1mol / L NaS to the filtrate 2 o 3 aqueous solution, and then adjust the pH to 3 with 1mol / L hydrochloric acid, extract with ethyl acetate, wash the organic phase with saturated NaCl solution, anhydrous NaCl 2 SO 4 Drying, concentration to dryness under reduced pressure, flash column chromatography, to obtain the title 2-butyl-4-chloro-1-[2'-(1-trityl-1H-tetrazol-5-yl)- 6.00 g of 1,1'-biphenyl-methyl]-imidazole-5-carboxylic acid, yield 88.2%.
[0046] 1 H-NMR (CDCl 3 , 400MHz): δ0.80(t, 3H), δ1.25(m, 2H), δ1.49(m, 2H), δ2.56(t, 2H), δ5.58(s, 2H), δ6 .94-7.54(m, 23H)
Embodiment 3
[0048] 2-Butyl-4-chloro-1-[2'-(1-trityl-1H-tetrazol-5-yl)-1,1'-biphenyl-methyl]-5-aldehyde -1H-imidazole
[0049] 37.3 g of 2-butyl-4-chloro-5-formyl-1H-imidazole, 4-bromomethyl-2'-(1-trityl-1H-tetrazol-5-yl)-biphenyl 111.5g, K 2 CO 3 56g and 800mL of anhydrous DMF were mixed, and the reaction was stirred at 90°C for 6h. Cool, add 1000g of ice water, and continue stirring for 1h. Extracted with ethyl acetate, washed the organic phase with water, washed with saturated NaCl solution, anhydrous NaCl 2 SO 4 Dry, concentrate under reduced pressure to a small volume, cool, place, precipitate solid, filter, wash with a small amount of ethyl acetate, and dry to obtain the title 2-butyl-4-chloro-1-[2'-(1-trityl 119.4 g of methyl-1H-tetrazol-5-yl)-1,1'-biphenyl-methyl]-5-hydroxymethyl-1H-imidazole, yield 90.0%.
[0050] 1 H-NMR (CDCl 3 , 400MHz): δ0.80(t, 3H), δ1.25(m, 2H), δ1.49(m, 2H), δ2.56(t, 2H), δ5.58(s, 2H), δ6 .94-7.54(m, 23H), δ9.61(s, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com