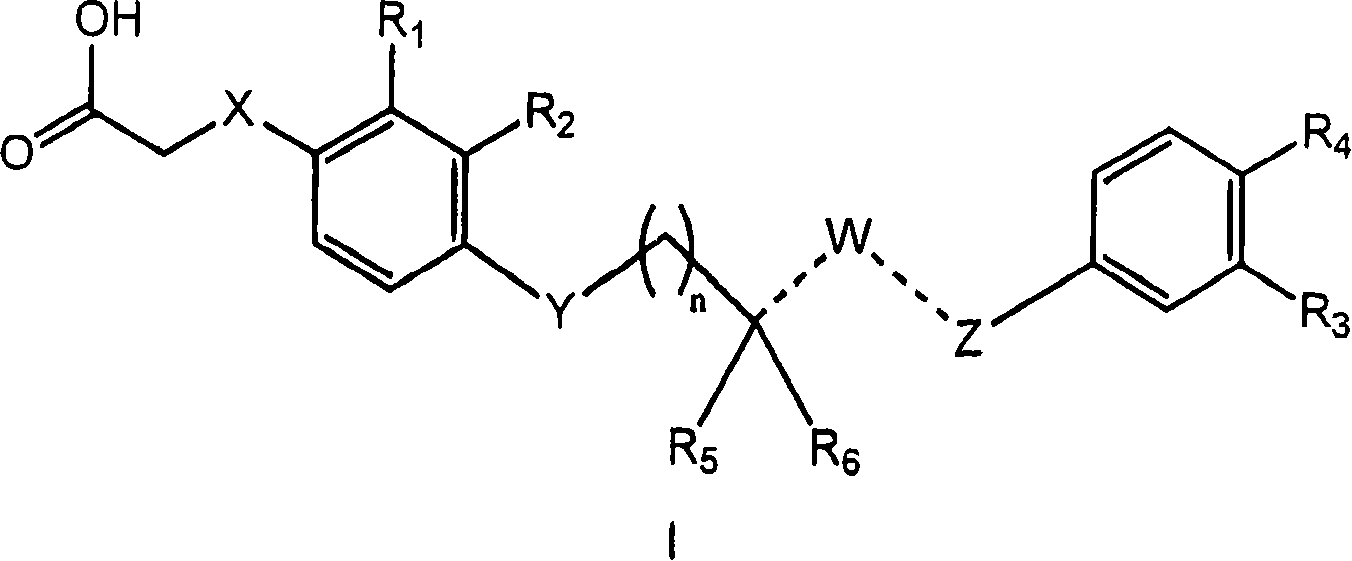
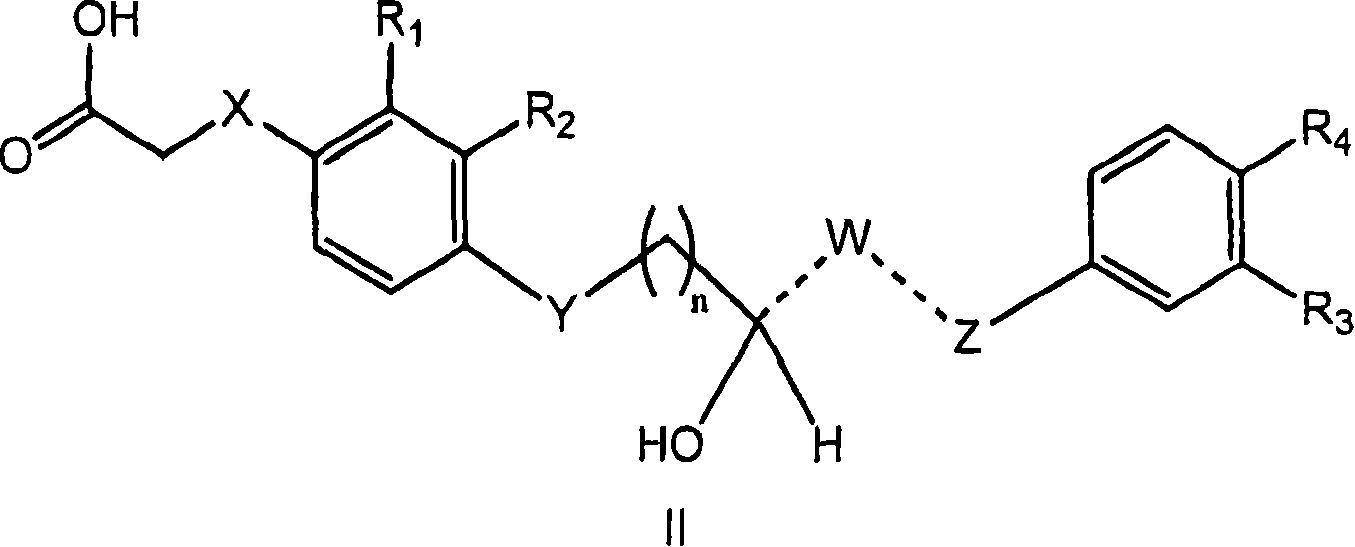
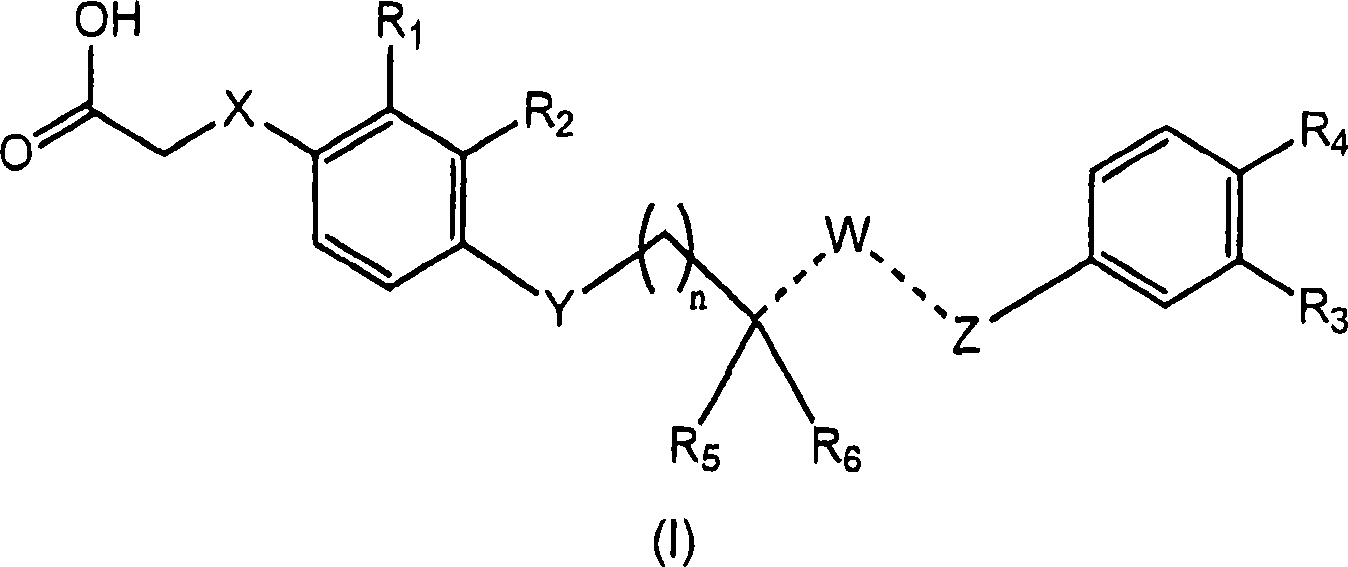
4-((phenoxyalkyl)thio)-phenoxyacetic acids and analogs
An alkyl and phenyl technology, applied in the field of 4-((phenoxyalkyl)thio)-phenoxyacetic acid and its analogs, can solve problems such as low efficacy and elevated HDL-C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0196] If mixtures of stereoisomers are obtained during the preparation of the compounds of the present invention, these isomers can be separated by conventional techniques such as preparative chromatography. The compounds may be prepared in racemic form, either by enantiospecific synthesis or by resolution of the individual enantiomers. Said compounds may be resolved into their individual component enantiomers by standard techniques such as salt formation to form diastereomers. The compounds can also be resolved by formation of diastereomeric esters or amides, followed by chromatographic separation and removal of the chiral auxiliary. Alternatively, the compounds can be resolved using a chiral HPLC column.
[0197] Examples of such synthetic routes include Examples 1-9. Compounds similar to the target compounds of these Examples can be prepared by similar routes. The disclosed compounds are useful in basic research and as pharmaceutical formulations as described in the fol...
Embodiment A
[0232]
[0233] Compound 1
[0234] {2-Methyl-4-[2-methyl-3-(4-trifluoromethyl-phenoxy)-propylthio]-phenoxy}-acetic acid
[0235] Process A1
[0236]
[0237] To a flask containing chlorosulfonic acid (15.0 ml, 226 mmol) was slowly added ethyl (2-methylphenoxy)acetate A1a (10.0 g, 51.6 mmol) at 4°C according to Scheme A1. The mixture was stirred at 4°C for 30 minutes and at room temperature for 2 hours, then poured into ice water. The precipitated white solid was filtered, washed with water, and dried in vacuo overnight to afford 14.0 g (93%, white solid) of A1b;
[0238] 1H NMR (300MHz, CDCl 3 )δ7.87-7.84(m, 2H), 6.80(d, J=9.5Hz, 1H), 4.76(s, 2H), 4.29(q, J=7.1Hz, 2H), 2.37(s, 3H), 1.31(t, J=7.1Hz, 3H); MS(ES) m / z: 315(M+Na+).
[0239] To a solution of Alb (4.70 g, 16.1 mmol) in EtOH (20 ml) was added 4.0 M HCl in dioxane (20 ml), and then 100 mesh tin powder (9.80 g, 82.6 mmol) was added in portions. The mixture w...
Embodiment B
[0255]
[0256] Compound 2
[0257] {2-Methyl-4-[2-(4-trifluoromethyl-phenoxymethyl)-butylthio]-phenoxy}-acetic acid
[0258] Process B
[0259]
[0260] To a solution of (S)-(+)-2-phenylbutyric acid B1 (352mg, 2.14mmol) in THF (3ml) at 0°C, 1.0M BH 3 • THF complex in THF (2.14ml, 2.14mmol). The mixture was allowed to warm to room temperature, stirred at room temperature overnight, quenched with water, then with 1.0N HCl, and washed with Et 2 O extraction (3 times). The extract was dried, concentrated and subjected to column chromatography to obtain 283 mg (88%) of B2;
[0261] 1H NMR (300MHz, CDCl 3 )δ7.34-7.29 (m, 2H), 7.24-7.16 (m, 3H), 3.70 (m, 2H), 2.65 (m, 1H), 1.79-1.67 (m, 1H), 1.63-1.48 (m, 2H), 0.82 (t, J=7.4Hz, 3H); MS (ES) m / z: 173 (M+Na+).
[0262] Add B2 (283mg, 1.88mmol), pyridine (0.76ml, 9.4mmol) and DMAP (23mg, 0.19mmol) in CH2Cl at 0°C 2 (3ml), acetyl chloride (369mg, 4.70mmol) was added. The mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com