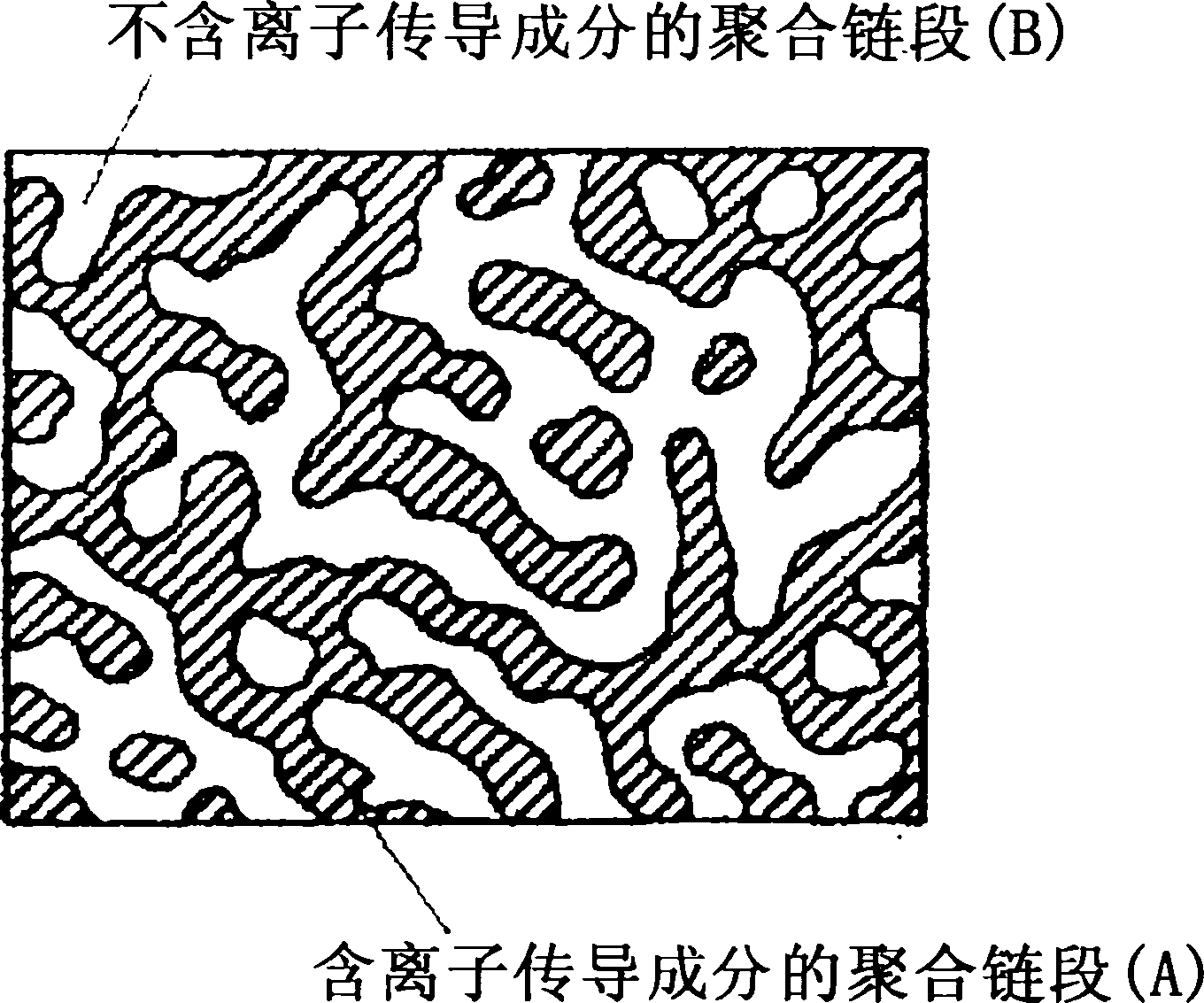
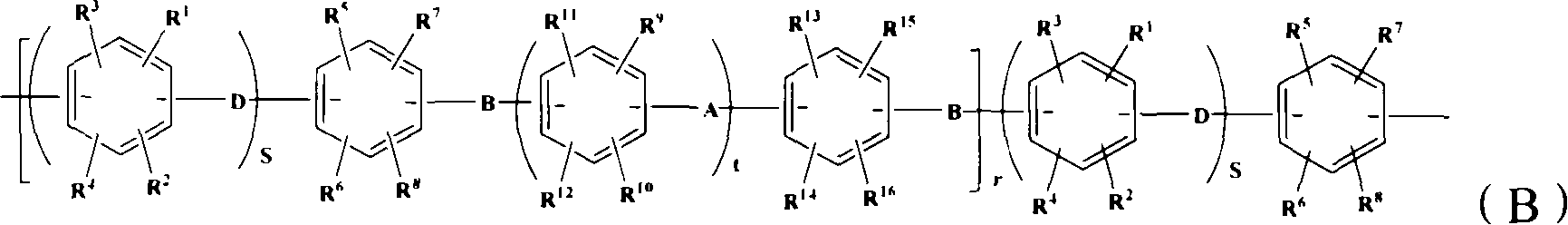
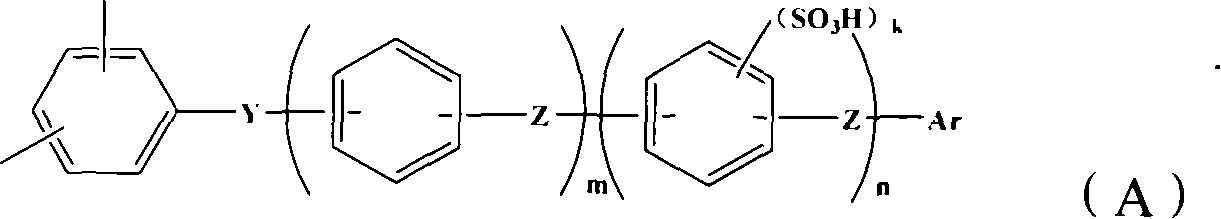Proton conductive membrane and its producing process
A technique for proton conducting membranes and manufacturing methods, applied in the field of proton conducting membranes, capable of solving problems such as decreased ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0212] (modulation of oligomers)
[0213] Add 67.3g (0.20 moles) of 2,2-bis(4-hydrobenzene)-1,1 to a 1L three-necked Erlenmeyer flask equipped with a stirrer, a thermometer, a cooling pipe, a Dean-Stark pipe, and a three-way valve for introducing nitrogen, 1,3,3,3-hexafluoropropane, 60.3g (0.24 mol) of 4,4'-dichlorobenzophenone (4,4'-DCBP), 71.9g (0.52 mol) of potassium carbonate, 300mL of N , N-dimethylacetamide (DMAc), and 150 mL of toluene were heated and stirred in an oil bath under a nitrogen atmosphere, and the reaction was carried out at 130° C. When the water and toluene generated by the reaction were azeotropically removed from the reaction system through the Dean-Stark tube to carry out the reaction, it was confirmed that almost no water was generated after about 3 hours. The reaction temperature was slowly increased from 130 to 150°C. Then, in the process of slowly increasing the reaction temperature to 150°C, remove most of the toluene, and continue the reaction ...
Synthetic example 2
[0219] Modulation of Polyarene Polymer Using Neopentyl as Protecting Group
[0220] Add 39.58g (98.64mmol) of 3-(2,5-Di Neopentyl benzenesulfonate, and 15.23 g (1.36 mmol) of the BCPAF oligomer (Mn=11200) prepared in Synthesis Example 1, 1.67 g (2.55 mmol) of Ni(PPh 3 ) 2 Cl 2 , 10.49g (40 mmol) of PPh 3 , 0.45 g (3 mmol) of NaI, 15.69 g (240 mmol) of zinc powder, 390 mL of dry NMP. Under the condition of stirring the reaction system, it was heated (finally heated to 75° C.) for 3 hours. Dilute the polymerization reaction liquid with 250 mL of THF, stir for 30 minutes, use celite as a filter aid, filter, and pour the filtered liquid into excess 1500 mL of methanol to make it solidify. The coagulum was collected by filtration, air-dried and redissolved in THF / NMP (200 / 300mL, respectively), and coagulated and precipitated with an excess of 1500mL methanol. After air-drying, 47.0 g (recovery rate 99%) of the desired yellow fibrous, neopentyl-protected, sulfone derivative co...
Synthetic example 3
[0227] (1) Synthesis of oligomers
[0228] Add 48.8g (284mmol) of 2,6-dichlorotoluonitrile and 89.5g (266mmol) of 2 to a 1L three-necked conical flask equipped with a stirrer, a thermometer, a cooling tube, a Dean-Stark tube, and a nitrogen introduction tube. 2-bis(4-hydrophenyl)-1,1,1,3,3,3-hexafluoropropane, 47.8 g (346 mmol) of calcium carbonate. After nitrogen replacement, 346 mL of sulfolane and 173 mL of toluene were added and stirred. The reaction solution was heated and circulated at 150° C. in an oil bath. The water produced by the reaction was collected with a Dean-Stark tube. When it was confirmed that almost no water was produced after 3 hours, toluene was removed from the reaction system through a Dean-Stark tube. The reaction temperature was gradually increased to 200° C., and after stirring was continued for 3 hours, 9.2 g (53 mmol) of 2,6-dichlorotoluonitrile was added, and the reaction was continued for 5 hours.
[0229] After the reaction solution was lef...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com