Novel polycrystal product of sertraline hydrochloride, and composition comprising same and new method for preparing same and non-formed anticle
A technology of sertraline hydrochloride and its crystal form, which is applied to new polymorphs of sertraline hydrochloride and compositions containing them, and can solve problems such as failure to obtain crystal form II of sertraline hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] According to the method for preparing sertraline hydrochloride crystal form II described in the '699 patent, we cannot obtain sertraline hydrochloride crystal form II. Therefore, obviously neither the '699 patent nor the '518 patent discloses a useful method for preparing sertraline hydrochloride.
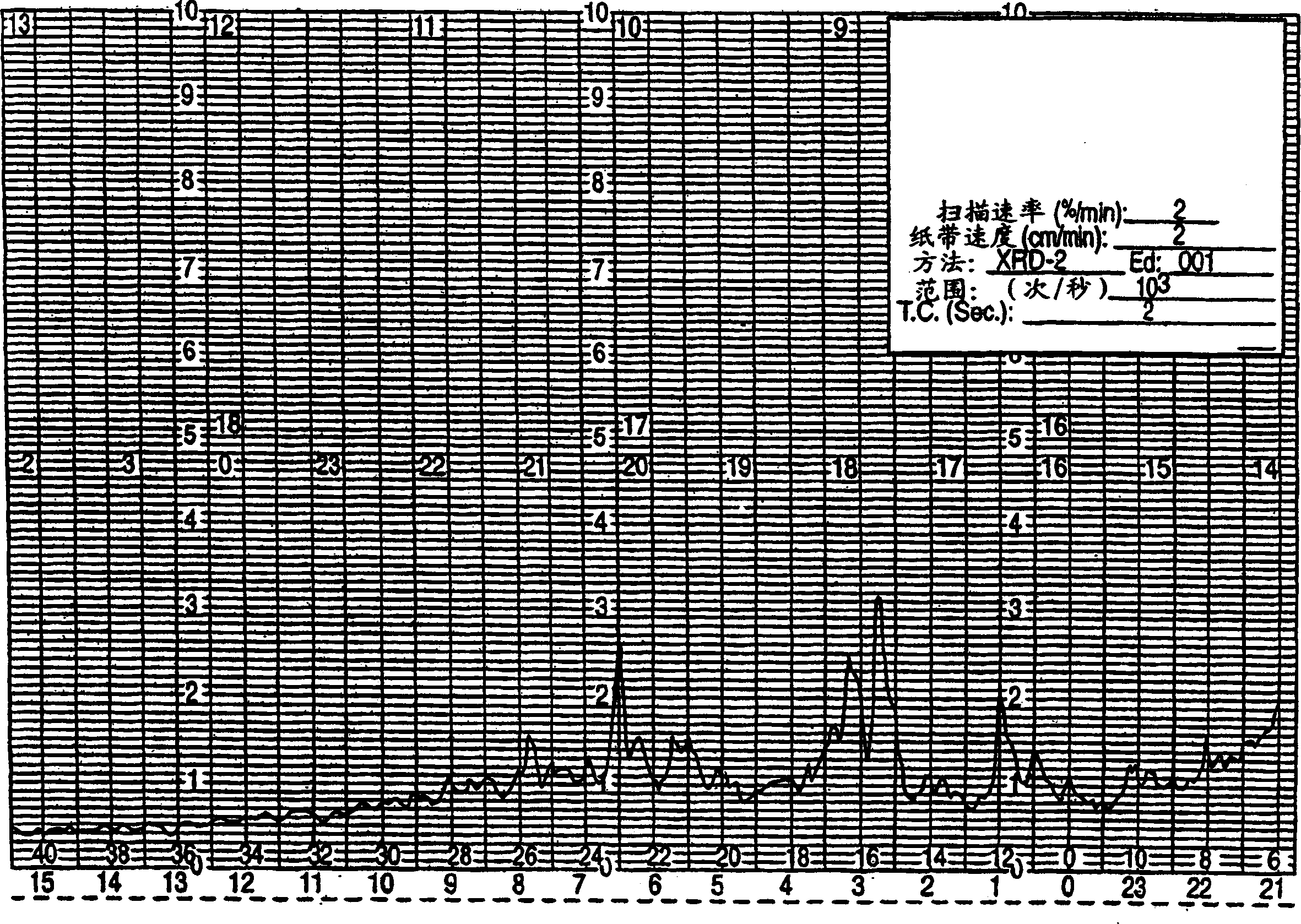
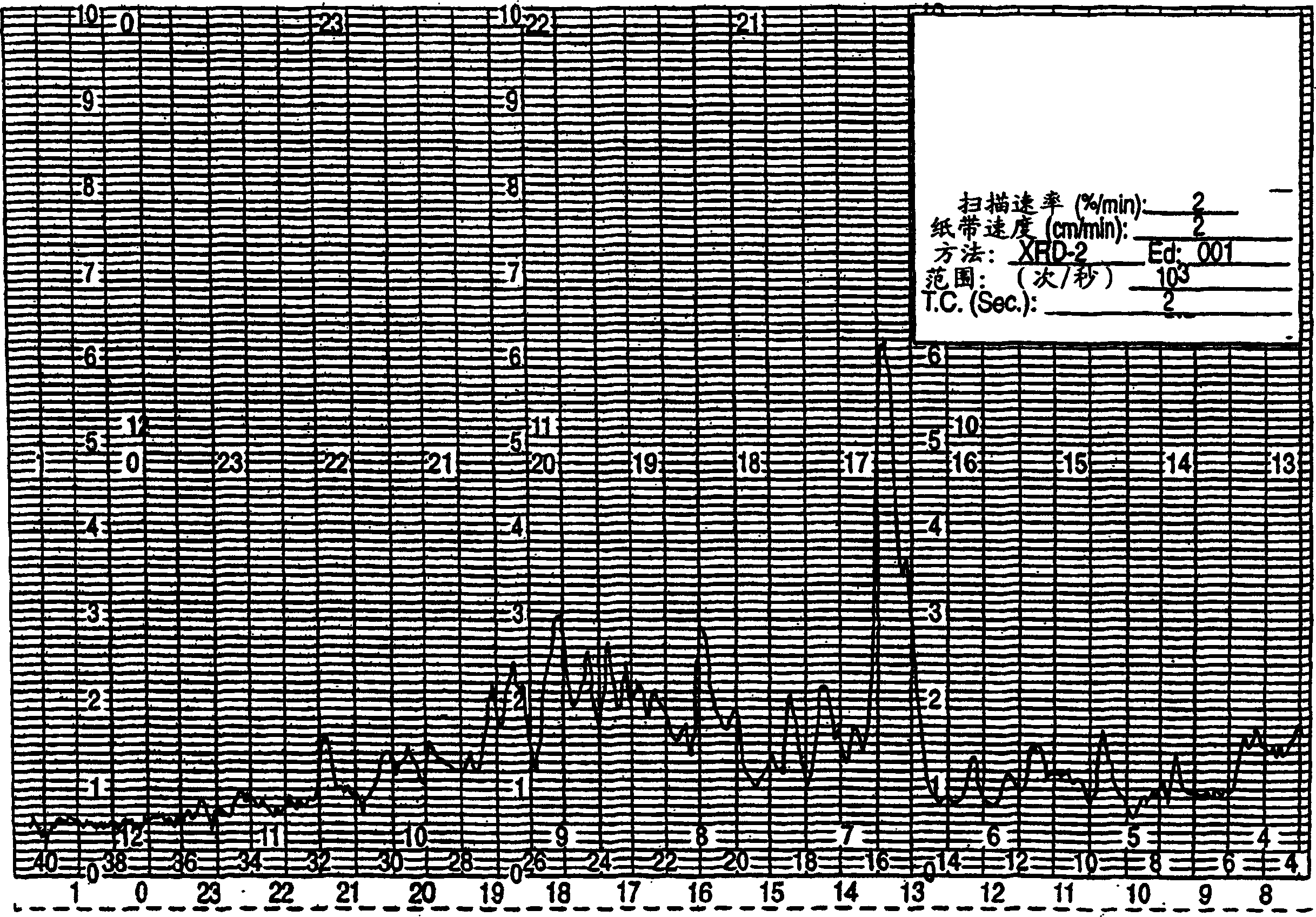
[0054] The invention also relates to a new method for preparing sertraline hydrochloride crystal form II through granulation. In the conversion of sertraline hydrochloride crystal form V to sertraline hydrochloride crystal form II, sertraline hydrochloride crystal form V is mixed with a small amount of ethanol or methanol. Stirring the mixture of sertraline hydrochloride crystal form V and ethanol or methanol for a period of at least several hours to several days, preferably about 2 days, promotes the conversion of crystal form V to crystal form II. Then the Sertraline Hydrochloride crystal form II was separated by filtration.
[0055] The present invention also provides a new m...
Embodiment 1
[0141] Preparation of Sertraline
[0142] Sertraline mandelate was prepared according to the method of US Patent No. 5,248,699. Sertraline mandelic acid (5 g) was stirred with 50 mL ethyl acetate at room temperature. The aqueous sodium hydroxide solution was added dropwise until sertraline mandelate was completely neutralized. Separate phases, organic phase passes MgSO 4 Dry and filter. The solvent was separated under reduced pressure to obtain an oil of sertraline base (3.2 g).
Embodiment 2
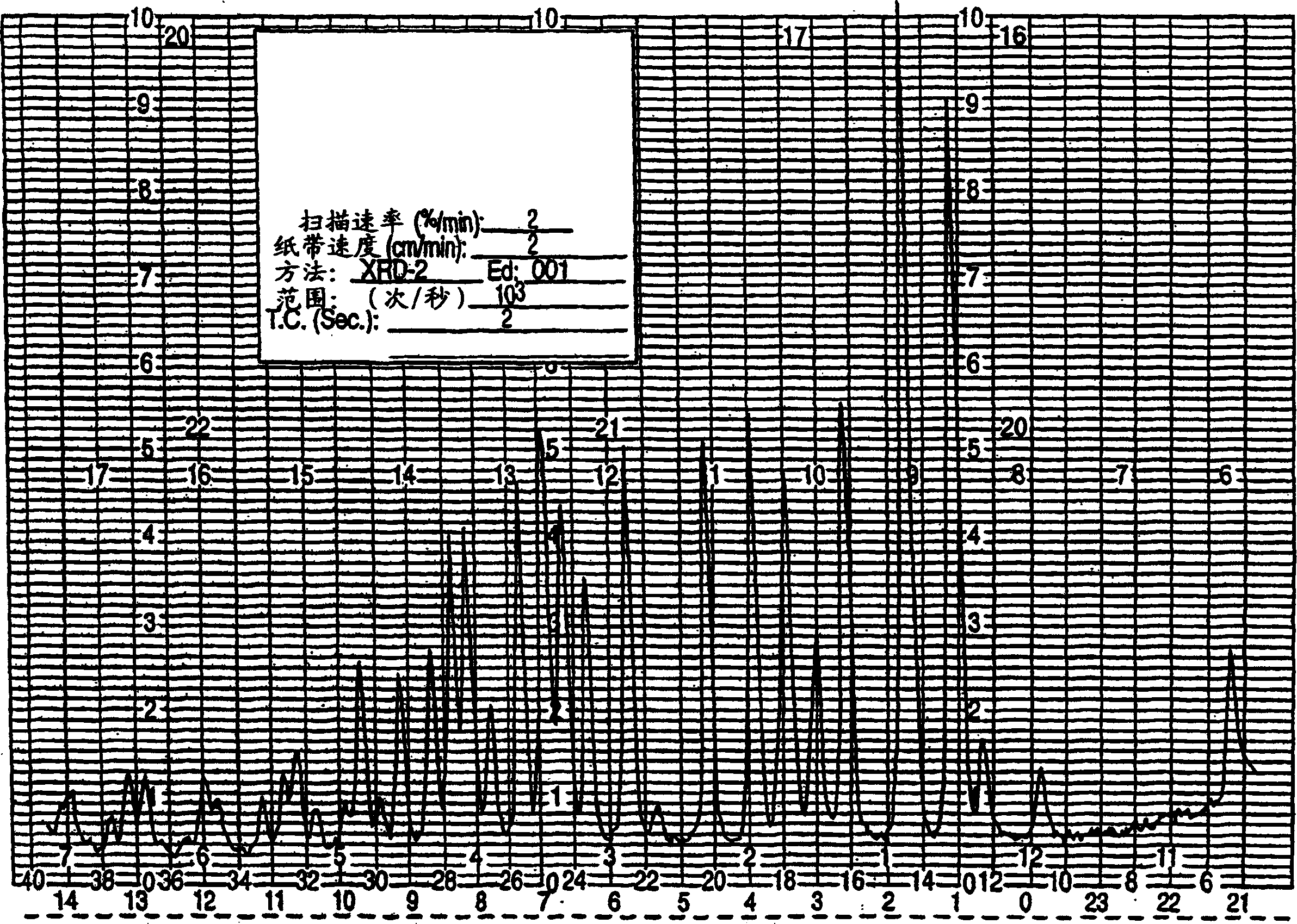
[0144] Preparation of Sertraline Hydrochloride Ethanolate Crystal Form VI by Re-beating Crystal Form I
[0145] Sertraline hydrochloride crystal form I (1 g) and absolute ethanol (20 mL) were stirred at room temperature for 24 hours. The mixture was filtered to obtain sertraline hydrochloride ethanolate form VI.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com