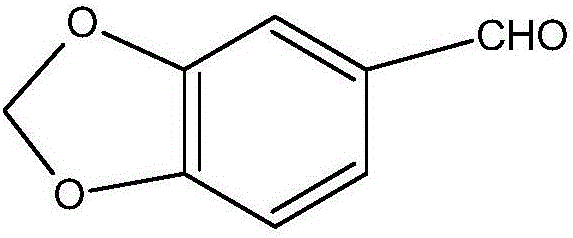
Reaction treatment method of heliotropin intermediate 3,4-dioxymethylene mandelic acid
A technology of methylene dioxybenzene and jasmine aldehyde, applied in the direction of organic chemistry, etc., can solve the problems of shortening the reaction time, speeding up the reaction speed, and difficulty in stirring, so as to shorten the reaction time, speed up the reaction speed, and solve the difficulty in stirring. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In a 2L reactor, first add 90g (0.5mol) of glyoxylic acid with a mass fraction of 40%, and add 100g (0.75mol) of piperonylcycline under stirring. Cool and control the temperature in the reactor to be 2 to 5°C; then add 150 g of catalyst concentrated sulfuric acid, and control the first reaction time to be 10 h; Control the temperature in the second reaction kettle to be 2-5°C, and the second reaction time is 3h. The reaction mixture is sampled and detected. When the content of piperonylcycline in the reaction mixture no longer decreases, the reaction is terminated; Add 285g of water, stir evenly, and then discharge and filter to obtain solid material and filtrate. The obtained solid material is 0.452mol of 3,4-methylenedioxymandelic acid, and the yield is 90.4%.
Embodiment 2-15
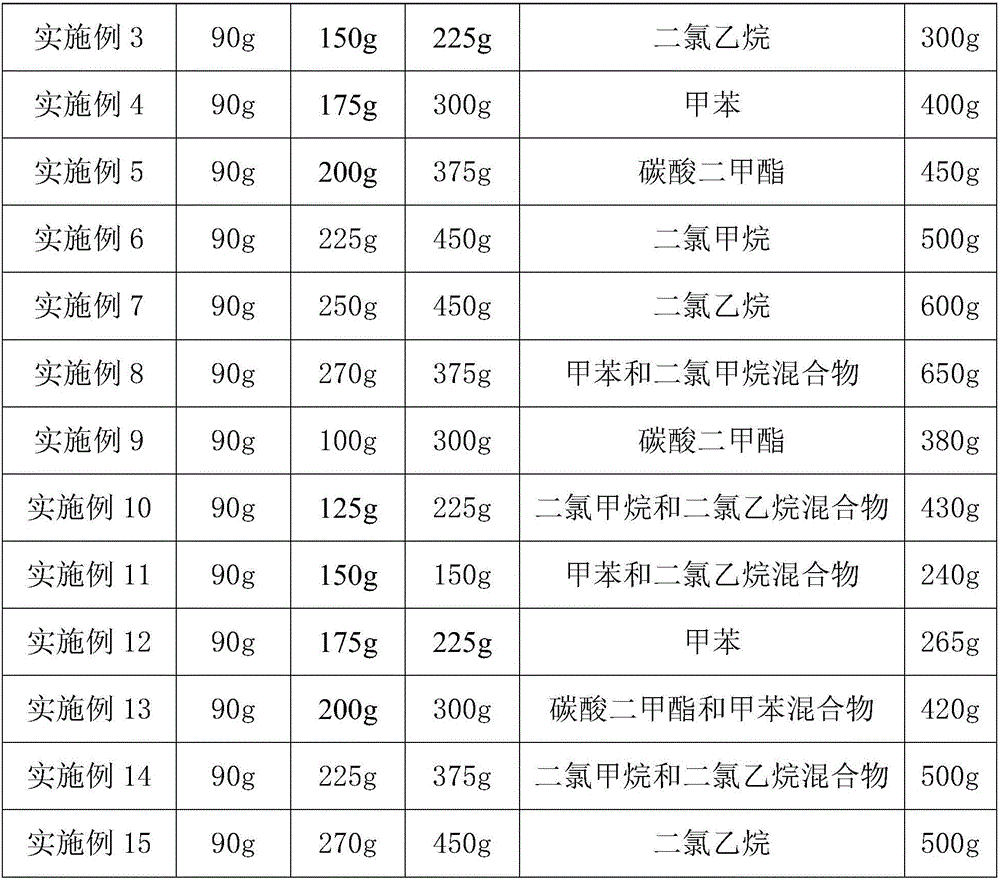
[0039] In Example 2-15, except changing the piperonylcycline, concentrated sulfuric acid, inert solvent, the first reaction time and the second reaction time, the reaction is carried out in the same manner as in Example 1. The specific formula is shown in Table 1, and the specific process conditions And the product yield is shown in Table 2.
[0040] Table 1
[0041]
[0042]
[0043] Table 2
[0044]
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com