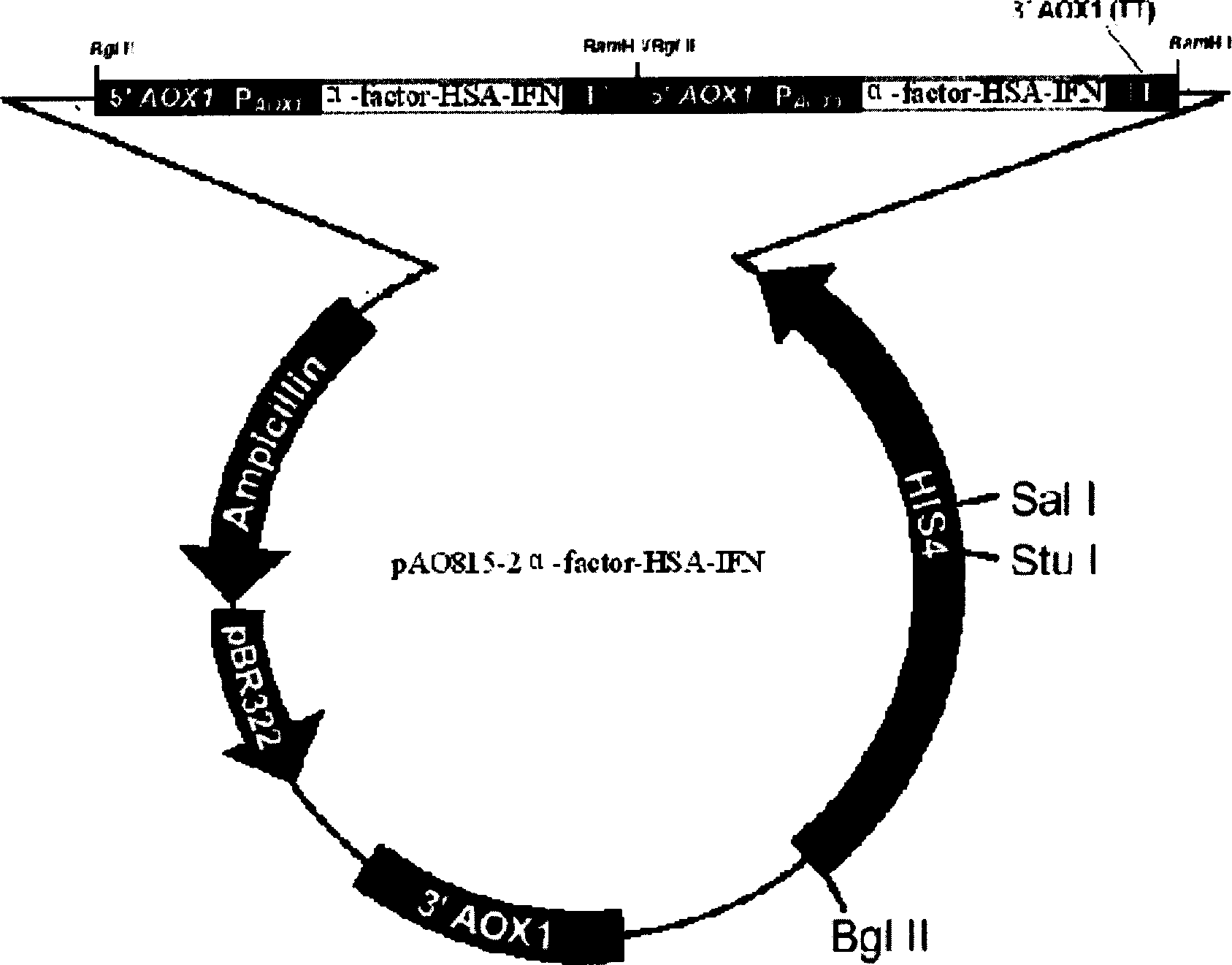
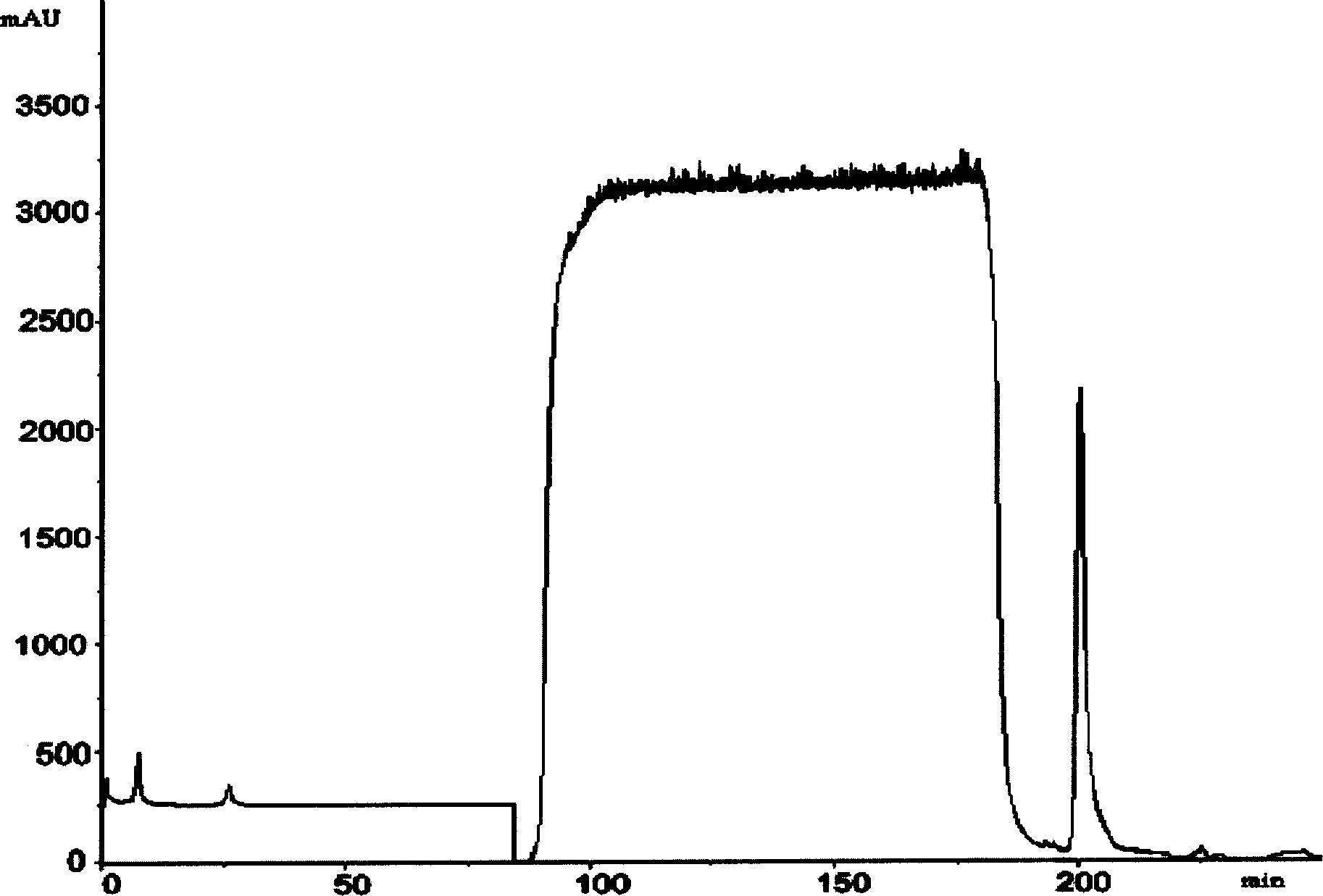
Preparation of human plasma protein fused long-effective interferon
A technology of human serum albumin and interferon, which is applied in the field of preparation of recombinant human serum albumin fused with long-acting interferon, can solve the problems of lack of literature and data on the influence of immunogenicity and therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention relates to a preparation method of fusion protein of human serum albumin and human interferon protein, the method at least includes the following steps:
[0025] (a) Cloning and codon optimization of human serum albumin gene;
[0026] (b) Human interferon alpha2b gene cloning and codon optimization;
[0027] (c) construction of human serum albumin gene and human interferon alpha2b gene connection and expression plasmid;
[0028] (d) Electrotransformation of Pichia pastoris and expression strain screening;
[0029] (e) fermentation culture; and,
[0030] (f) Fusion protein purification.
[0031] Wherein, in step (a), HSA cDNA is obtained from human fetal liver cDNA library by PCR method. In addition to including the 5' end sequence of the HSA gene, ALBF also added a Kex2 restriction signal recognition sequence (underlined part) before its 5' end:
[0032] ALBF: 5' AAAAGA GATGCACACAAGAGTGAGGTTGC3'
[0033] ALBB: 5'TAAGCCTAAGGCAGCTTGACTTGC3'
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com