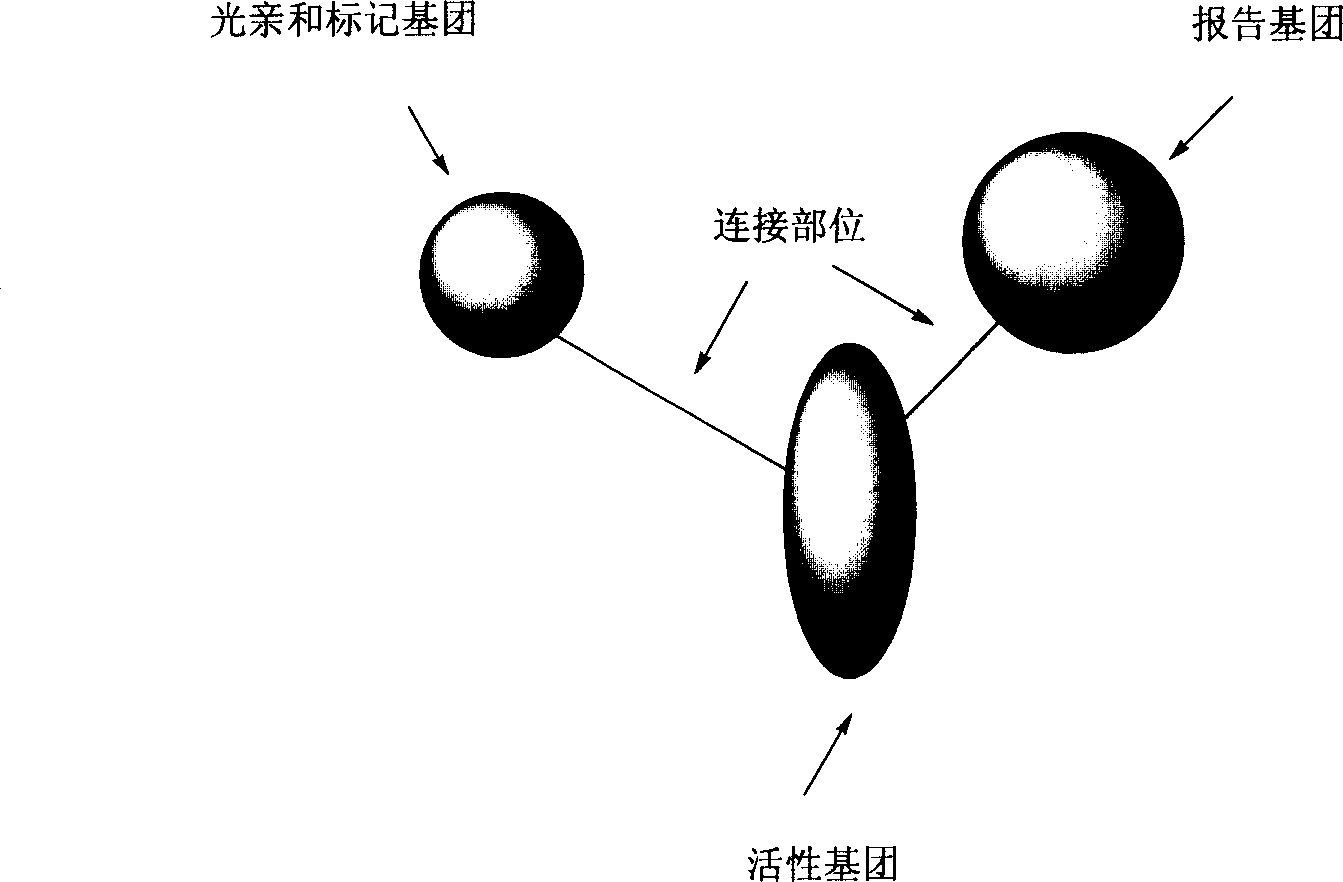
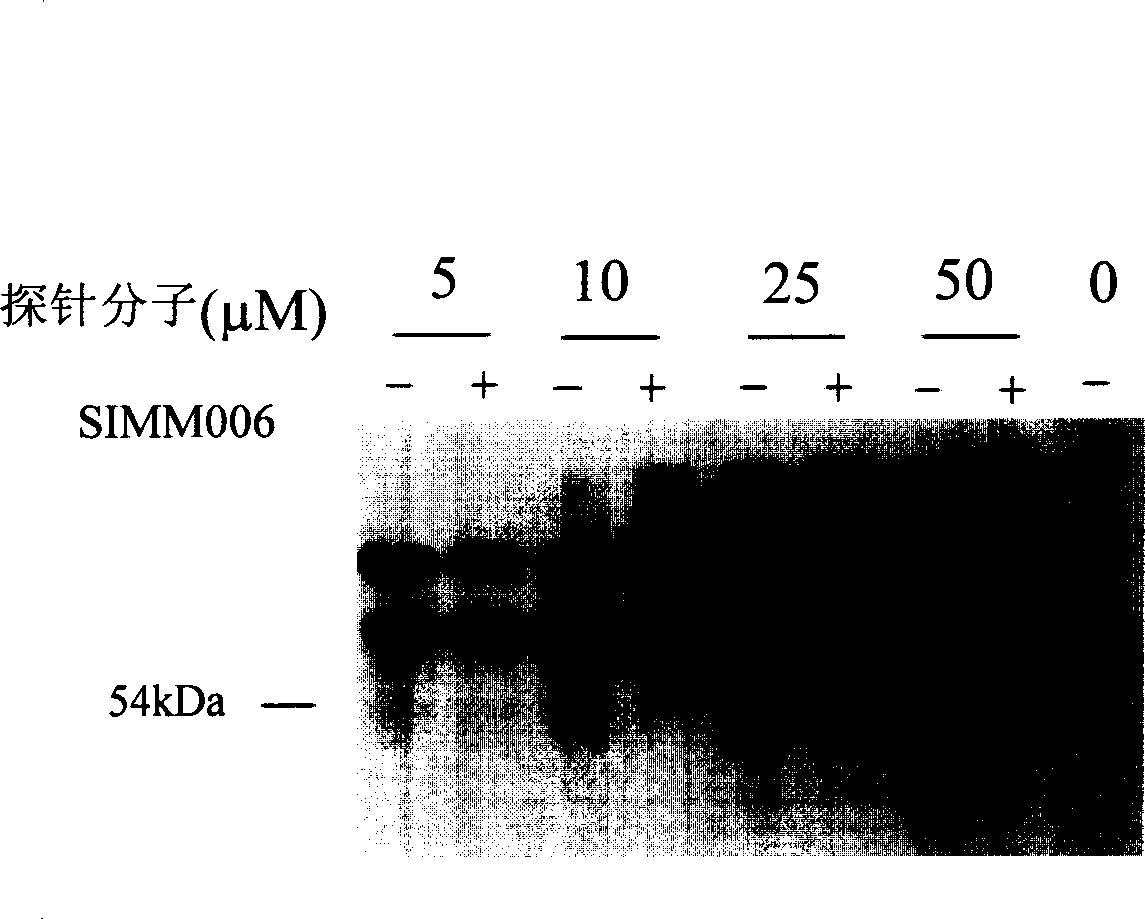
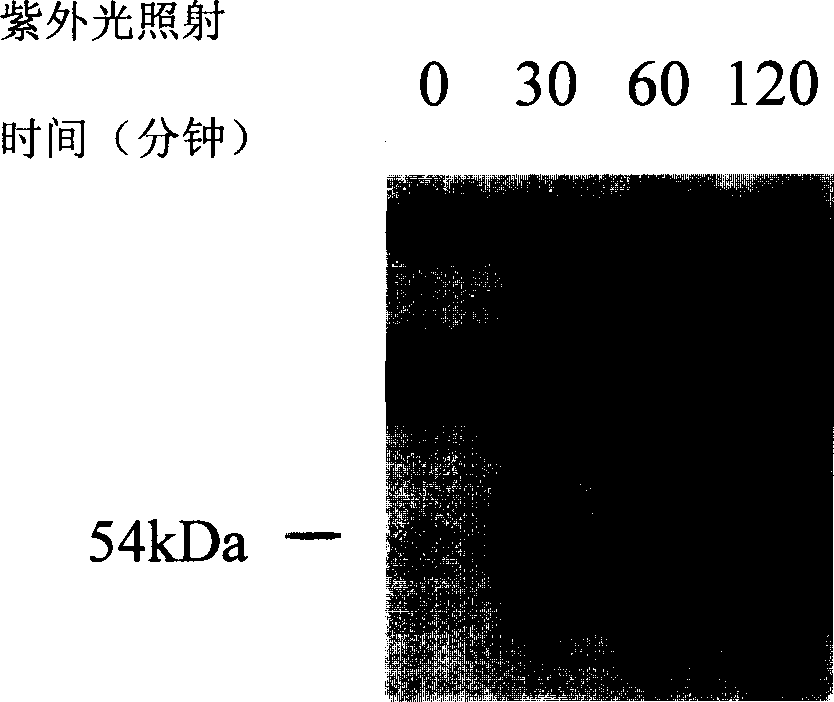
One class of light affinity labelling double function probe molecules, preparing and use
A probe molecule and bifunctional technology, applied in a class of photoaffinity labeled bifunctional probe molecules, preparation and application fields, can solve a wide range of problems such as incomplete activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The following examples illustrate the probe molecules covered by the present invention and their design, preparation, and application, but here are only examples and do not limit the present invention.
[0048] Preparation of compound 1:
[0049]
[0050] Paeoniflorin (0.720g, 1.5mmol) and imidazole (1.224g, 18.00mmol) were dissolved in 30mL DMF, and TBSCl (1.356g, 9.00mmol) was added at 0°C. After stirring at room temperature for 12 hours, the reaction was completed, and 30 mL of H 2 O, extracted with 3×50mL ether, combined the organic layers, washed with 3×30mL saturated brine, separated the organic layer, anhydrous Na 2 SO 4 dry. Silica gel column chromatography (petroleum ether: ethyl acetate = 3:1) gave 0.670 g of a colorless oily substance, with a yield of 63.0%.
[0051] 1 H NMR (300MHz, CD3OD): δ0.92(18H), 0.11(12H), 1.36(3H), 1.82(1H), 1.90(1H), 2.09(1H), 2.52(1H), 2.59(1H), 3.12-3.38(3H), 3.68(1H), 3.95(1H), 4.48(1H), 4.75(2H), 5.42(1H), 7.48(2H), 7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com