Pharmaceutical use of anti-TLR4 antibody
A technology of use and antibody, applied in the field of pharmaceutical use of anti-TLR4 antibody, can solve the problem of unclear role of soluble partial receptors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0164] in vitro studies
[0165] Materials and methods
[0166] proteins and toxins
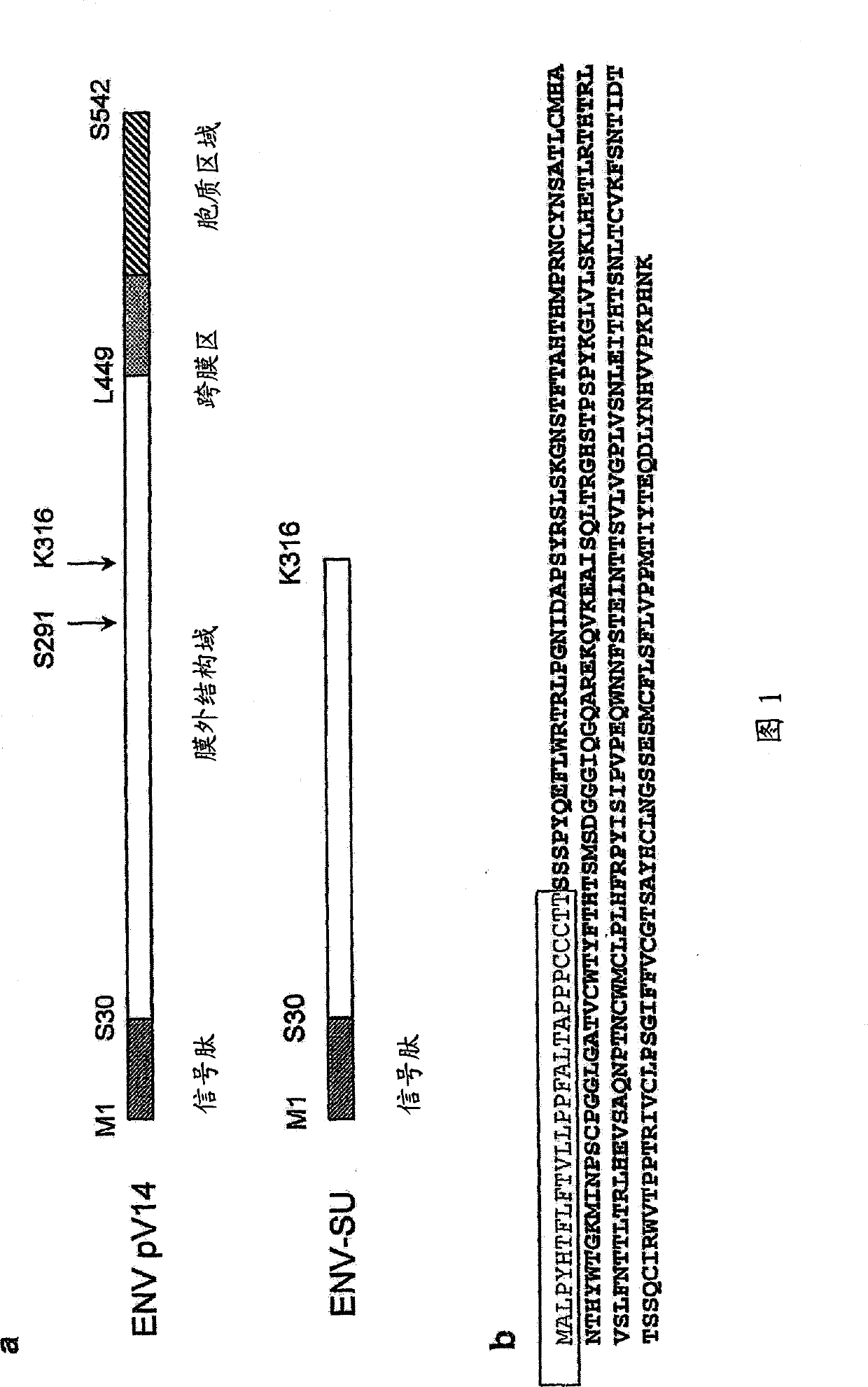
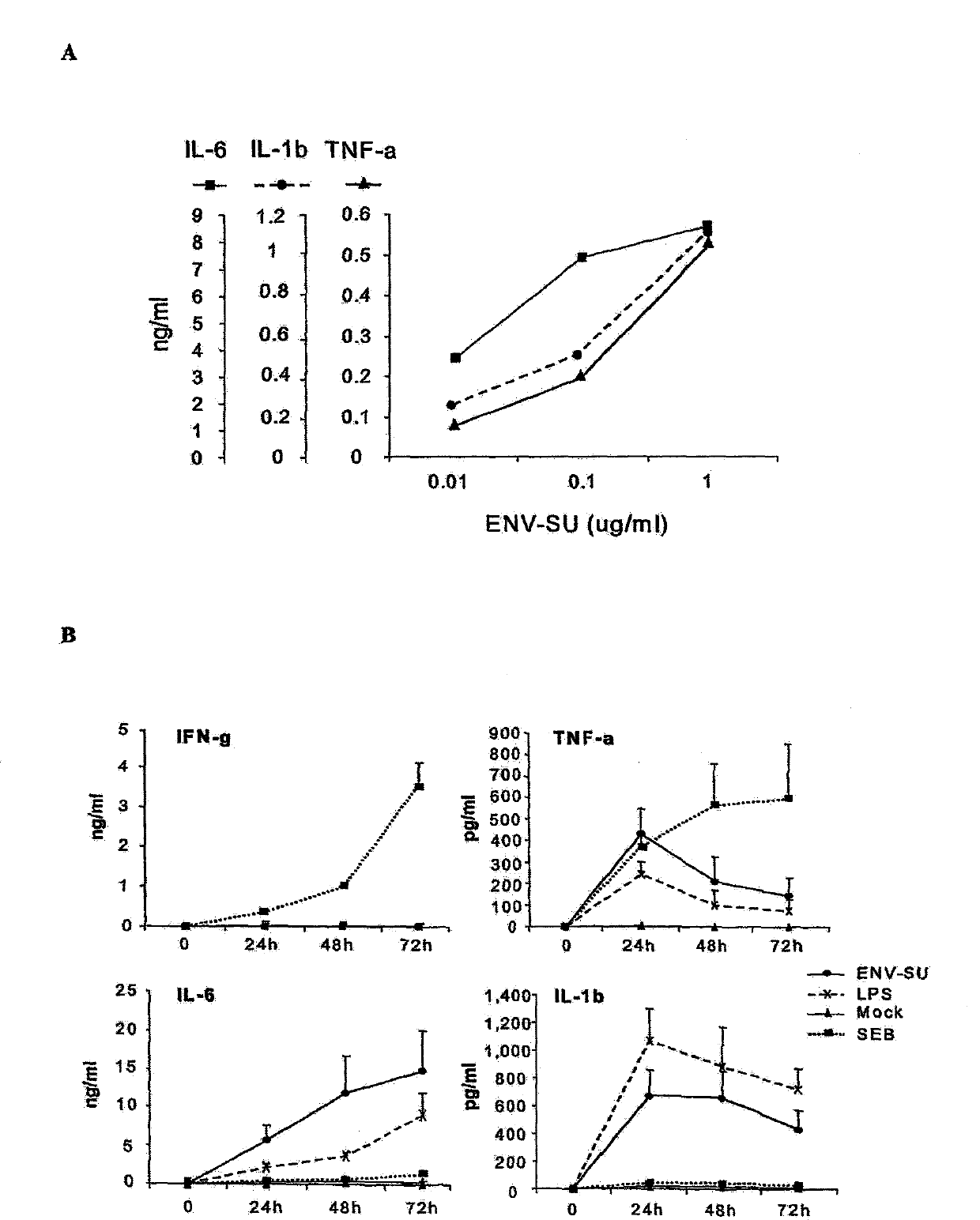
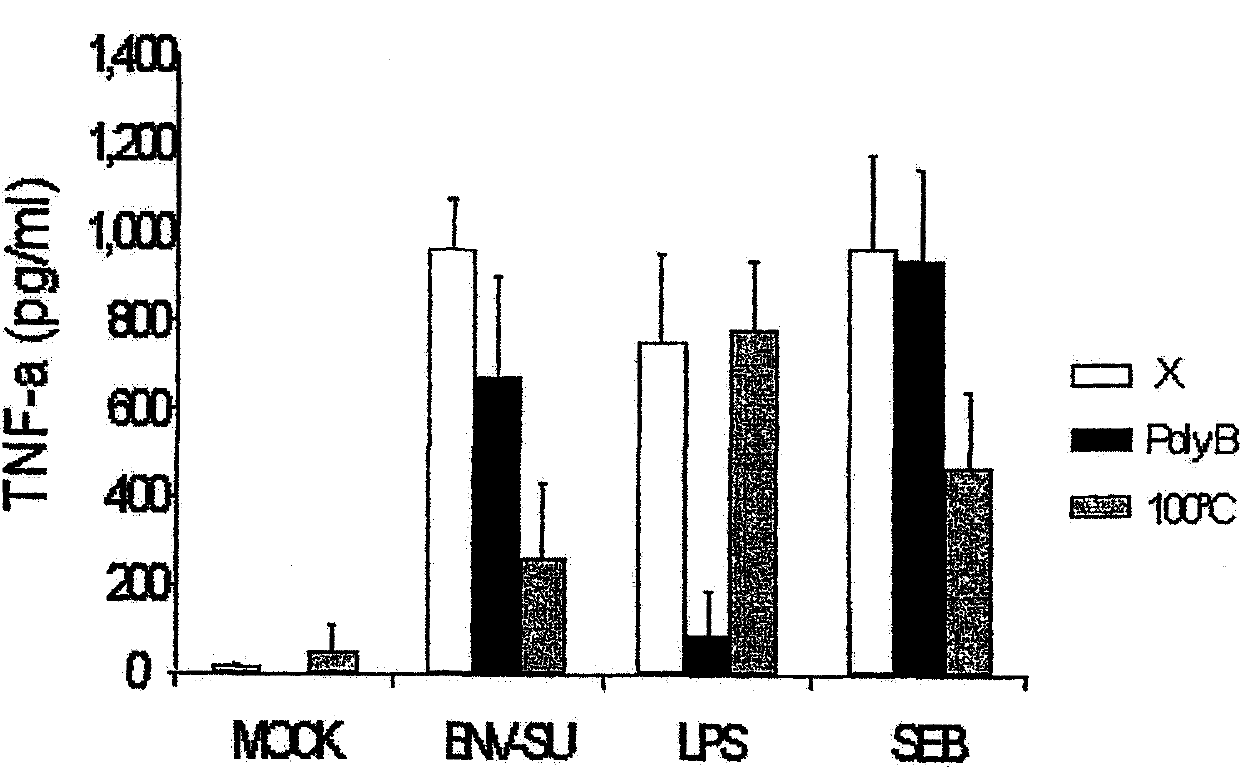
[0167] The surface protein of the MSRV envelope (Env-SU) corresponds to the protein sequence of 287 amino acids of the total envelope protein (Env Pv14, GenBank AF331500). The structures and amino acid sequences of EnvPv14 and ENV-SU are shown in Figures 1(a) and 1(b), respectively. The recombinant MSRVEnv-SU protein was expressed in E. coli and purified on an FPLC column. The quality and purity of the protein were further confirmed by mass spectrometry and Western blotting. Casein kinase was used as an autologous negative control. This control protein was produced and purified under the same conditions as Env-SU.
[0168] Detection of the presence of endotoxin in both proteins was performed by the Limulis amebocyte lysate (LAL) assay by the company CleanCells (Bouffere, France). All fractions were below the detection threshold of 5 IU / ml. Staphylococcal endotoxin was obtained from ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com