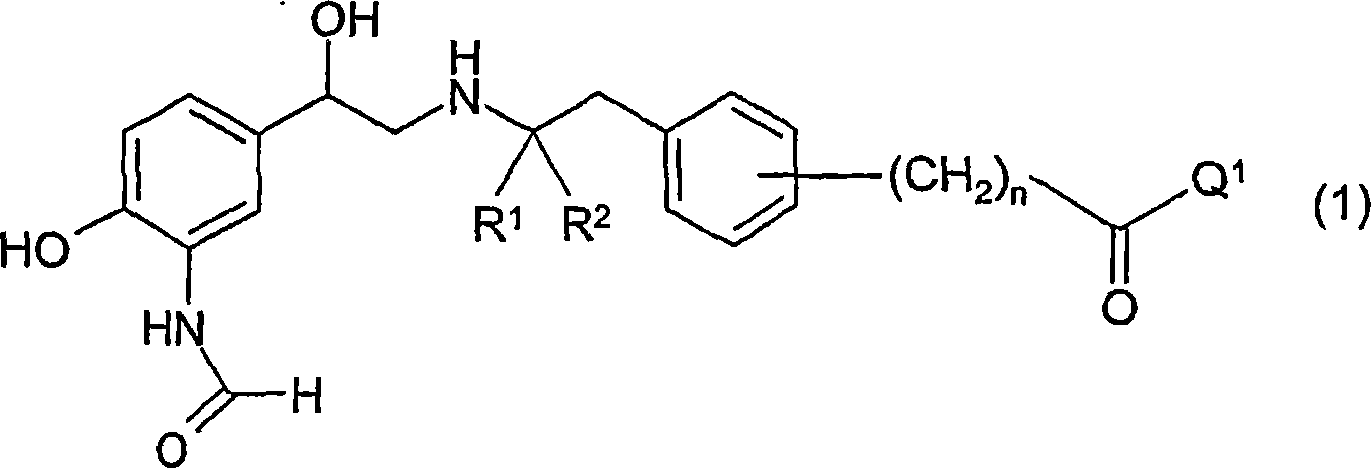
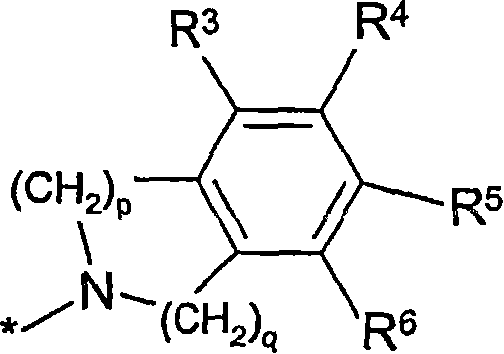
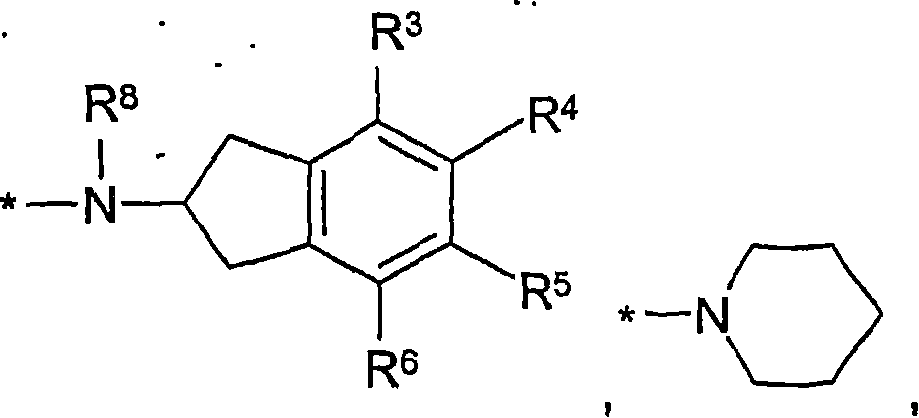
Formamide derivatives useful as adrenoceptor
A halogen, compound technology, applied in the field of β2 agonists, can solve the problem of not showing pharmacological profile, etc., to achieve the effect of excellent potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0810] Example 1: N-benzyl-2-(3-{2-[(2R)-2-(3-formylamino-4-hydroxyphenyl)-2-hydroxyethylamino]-2-methyl Propyl}phenyl)acetamide
[0811]
[0812]Preparation 15 (24 mg, 40 micromol), formic acid (0.5 mL), tetrahydrofuran (5 mL) and water (0.5 mL) were heated to 90° C. for 18 hours. Further aliquots of formic acid (0.5 mL) and tetrahydrofuran (5 mL) were added and heating continued for a further 18 hours. Solvent was removed and chromatographed (0-10% methanol in dichloromethane + 0.3% ammonia). The product was dissolved in methanol (x3) and evaporated (10 mg).
[0813] 1 HNMR (CD 3 OD, 400MHz) δ: 1.05-1.09 (6H, m), 2.69-2.78 (2H, m), 2.78-2.83 (1H, m), 2.87-2.93 (1H, m), 3.53 (2H, s), 4.35 (2H,s), 4.65(1H,dd), 6.82-6.86(1H,m), 6.99(1H,dd), 7.03-7.06(1H,m), 7.13-7.28(7H,m), 8.08(d ), 8.28(s), 8.55(s), 8.61(s); MS(APCI) m / z 476[M+H] + ; HRMS C 28 h 33 N 3 o 4 476.2544[M+H] + found 476.2533.
Embodiment 2
[0814] Example 2: N-(3,4-dimethylbenzyl)-2-(3-{2-[(2R)-2-(3-formamido-4-hydroxyphenyl)-2-hydroxy Ethylamino]-2-methylpropyl}phenyl)acetamide:
[0815]
[0816] The preparation was carried out using the amide from preparation 16 and the method described for Example 1 .
[0817] 1 HNMR (CD 3 OD, 400MHz) δ: 1.05-1.08 (6H, m), 2.18 (3H, s), 2.19 (3H, s), 2.67-2.94 (4H, m), 3.52 (2H, s), 4.27 (2H, s ), 4.62(1H,dd), 4.65(1H,dd), 6.81-7.06(6H,m), 7.12-7.24(3H,m), 8.07(d), 8.27(s), 8.55(s), 8.61 (s).
[0818] MS(APCI)M / Z 504[M+H] + ; HRMS C 30 h 37 N 3 o 4 504.2857[M+H] + FOUND504.2842.
Embodiment 3
[0819] Example 3: N-[4-(dimethylamino)benzyl]-2-{3-[(2R)-2-({(2R)-2-[3-(formylamino)-4- hydroxyphenyl]-2-hydroxyethyl}amino)propyl]phenyl}acetamide
[0820]
[0821] A mixture of the product from Preparation 27 (131 mg, 0.21 mmol) and triethylamine trihydrofluoric acid (16 μL, 0.10 mmol) in THF (2 mL) was stirred at room temperature for 3 days. The mixture was then concentrated in vacuo, and the residue was purified by column chromatography on silica gel (eluting with dichloromethane:methanol:0.88 ammonia, 100:0:0 to 90:10:1) to yield 36% Yield The title compound as a brown foam, 18 mg.
[0822] 1 H NMR (400MHz, CD 3 Cl 3 )δ: 1.07 (3H, m), 2.57 (1H, dd), 2.67-2.76 (2H, m), 2.85-2.99 (2H, m), 2.87 (6H, s), 3.47 (1H, m), 4.23 (2H,s), 4.68(1H,dd), 6.67-6.71(2H,m), 6.77-6.79(2H,d), 6.90(1H,m), 6.97-7.70(m,6H), 7.97(d ), 8.27(s), 8.35(s); LRMS APCI m / z 619[M+H] -
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com