Alprostadil preparation and its making method
A technology for alprostadil and preparations, applied in the field of preparation of alprostadil preparations, can solve the problems of complex preparation process, vascular irritation and the like, and achieve the effects of simple process, improved stability and improved lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
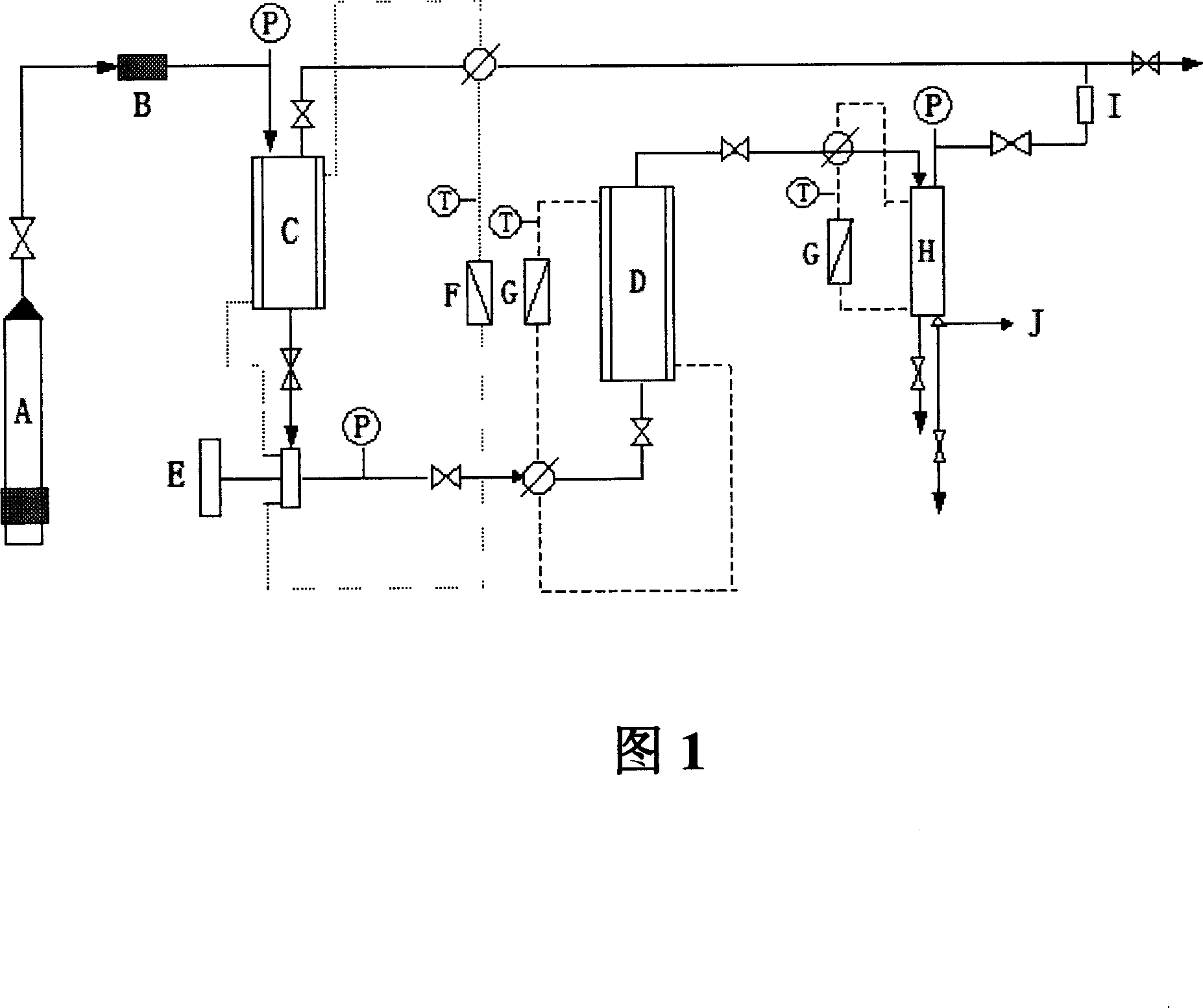
[0070] Figure 1 is a flowchart of the dissolution of alprostadil by supercritical carbon dioxide (reference: Hou Yucui, Sun Junhong, Solubility of Egg Oil in Supercritical Carbon Dioxide and Its Simulation, 2000, 14(3), 62-67).
[0071] First put 10g of alprostadil (Biochemical Branch of Bethune Medical University Pharmaceutical Factory) into the extractor, open the inlet valve of the extractor, and introduce CO 2 , so that CO 2 The pressure is equilibrated and the CO is turned on 2 Metering pump, when the pressure of the extractor reaches the required pressure, open the regulating valve at the outlet of the extractor to maintain the required pressure, periodically release alprostadil through a special nozzle at the bottom of the separator, and weigh and calculate. The temperature is controlled by a water bath with an accuracy of 0.5°C; the pressure is controlled by a throttle valve with an accuracy of 0.5MPa; the flow rate is controlled by CO 2 Metering pump control, the ma...
Embodiment 2
[0082] 2. Preparation process
[0083] 500mg of alprostadil is loaded into the extractor, the temperature is constant at 30°C, the pressure is 30MPa, CO 2 The flow rate is kept constant at 150-200L / min for 90 minutes. After alprostadil is dissolved in supercritical carbon dioxide, open the outlet regulating valve of the extractor, keep the pressure at 30MPa, open the nozzle at the bottom of the separator, so that alprostadil is ejected in the form of ultrafine particles, and weigh 20mg, 30mg, and 100mg respectively , respectively add 1000ml of water for injection, 50ml of phosphate buffer saline and 300ml of auxiliary material 10% dextran mixed in proportion in advance, and stir evenly. Sterilize by filtration with a 0.2 μm pore size filter membrane, and fill it after checking the clarity. Each 1000 vials of the drug solution were divided into vials and freeze-dried. Check the packaging.
Embodiment 3
[0086] 2. Preparation process
[0087] Alprostadil 500mg is loaded into the extractor, the temperature is constant at 35°C, the pressure is 30Mpa, CO 2 The flow rate is kept constant at 150-200L / min for 90 minutes. After alprostadil is dissolved in supercritical carbon dioxide, open the outlet regulating valve of the extractor, keep the pressure at 30MPa, open the nozzle at the bottom of the separator, so that alprostadil is ejected in the form of ultrafine particles, and weigh 20mg, 30mg, and 100mg respectively, Add 1000ml of water for injection, 50ml of phosphate buffer saline and 300ml of auxiliary material 10% dextran which have been mixed in proportion in advance, and stir evenly. Sterilize by filtration with a 0.2 μm pore size filter membrane, and fill it after checking the clarity. Each 1000 vials of the drug solution were divided into vials and freeze-dried. Check the packaging.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com