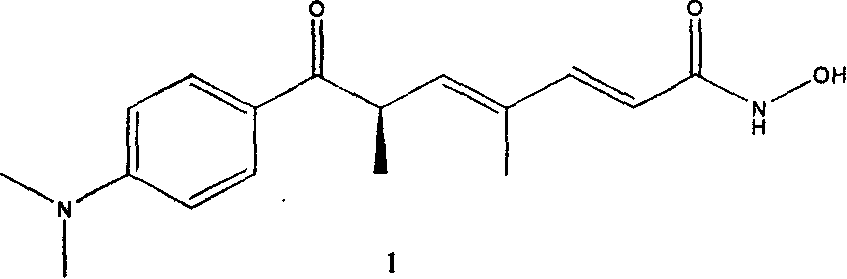
Chiral synthesis of combined protein deacetylated enzyme inhibitor
A technology of deacetylase and chiral synthesis, applied in the direction of organic chemistry, etc., can solve the problems of low overall yield, expensive, poor stability of intermediates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
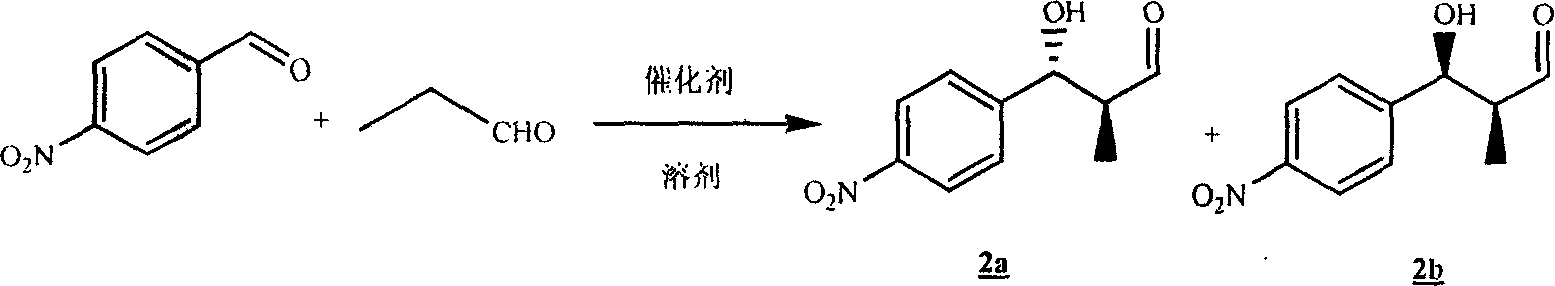
[0060] Embodiment one: compound 2a and 2b Preparation of:
[0061] Dissolve 1.51g of p-nitrobenzaldehyde in 35mL of DMF, add 230mg of L-proline, and then add 1.44mL of propionaldehyde, stir at room temperature for 7 hours, and follow the complete reaction by TLC. Add 100mL of water, extract three times with ethyl acetate, combine the organic layers, dry over magnesium sulfate, and dry the solvent with a vacuum pump to obtain 2a and 2b The crude product was directly put into the next step.
[0062] compound 2a The hydrogen spectrum:
[0063] 1 HNMR (300MHz, CDCl 3 ): δ9.802 (1H, d, J = 0.9Hz), 8.224 (2H, d, J = 6.6Hz), 7.548 (2H, d, J = 6.3Hz), 4.974 (1H, d, J = 6.0Hz ), 3.409 (1H, br), 2.774 (1H, m), 0.996 (3H, d, J=5.7Hz).
Embodiment 2
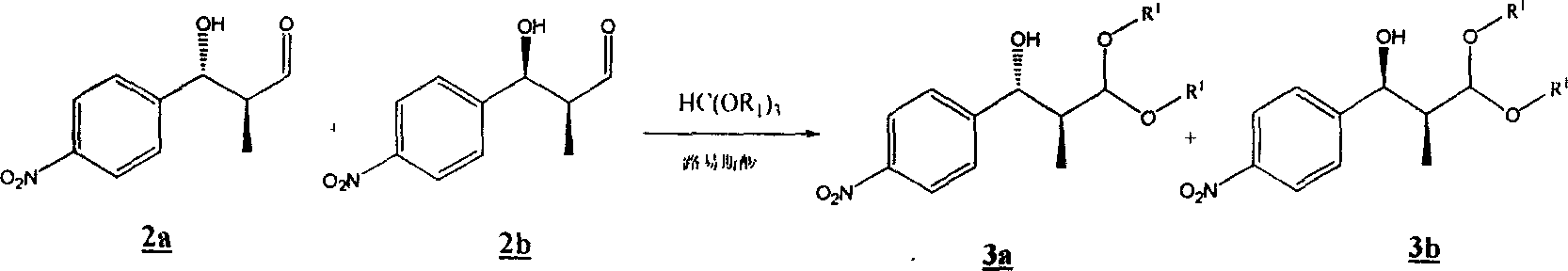
[0064] Embodiment two: compound 3a and 3b Preparation of:
[0065] will contain 2a and 2b The crude product was added with 30 mL of methanol, 3.00 g of trimethyl orthoformate and 380 mg of p-toluenesulfonic acid monohydrate, and stirred at room temperature for 30 minutes. Add 10 mL of saturated sodium bicarbonate solution and 100 mL of water, extract three times with ethyl acetate, combine the organic layers, dry over magnesium sulfate, and drain the solvent to obtain a crude product. The compound was purified by column chromatography 3a and 3b A total of 2.26g ( 1 Proof of HNMR analysis 3a : 3b =11:1), the two-step total yield was 88.6%.
[0066] compound 3a The hydrogen spectrum:
[0067] 1 HNMR (300MHz, CDCl 3 ): δ8.202 (2H, d, J = 8.7Hz), 7.513 (2H, d, J = 8.7Hz), 4.719 (1H, d, J = 8.4Hz), 4.313 (1H, d, J = 5.7Hz ), 3.497(3H, s), 3.416(3H, s), 2.114(1H, m), 0.686(3H, d, J=7.2Hz);
[0068] compound 3b The hydrogen spectrum:
[0069] 1 HNMR (400MHz, CDCl...
Embodiment 3
[0073] Embodiment three: compound 4a and 4b Preparation of:
[0074] 3.63g 4a and 4b The mixture was dissolved in 25 mL DMF with 4.28 g TBSCl and 2.90 g imidazole, and stirred at room temperature for 20 hours. Add 80mL of water, extract with ethyl acetate, and the organic layer is dried with magnesium sulfate and pumped dry to obtain 4a and 4b crude product. Separation by column chromatography 4a and 4b , to get the compound 4a A total of 3.75 g, as a light yellow solid, yield 71.4%.
[0075] 1 HNMR (300MHz, CDCl 3 ): 68.173 (2H, d, J = 8.7Hz), 7.467 (2H, d, J = 8.7Hz), 4.804 (1H, d, J = 6.6Hz), 4.152 (1H, d, J = 5.4Hz), 3.376(3H, s), 3.365(3H, s), 0.883(9H, s), 0.689(3H, d, J=6.9Hz), 0.053(3H, s), -0.236(3H, s);
[0076] MS(EI)m / z(%): 369[M + ], 266(35), 240(100), 89(11);
[0077] [α] D 22 +35.3° (c=2.20, CHCl 3 ).
[0078] Mp 35-36°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com