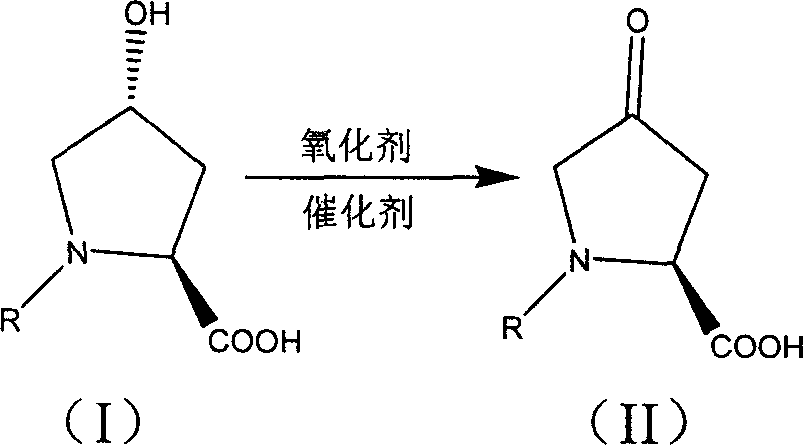
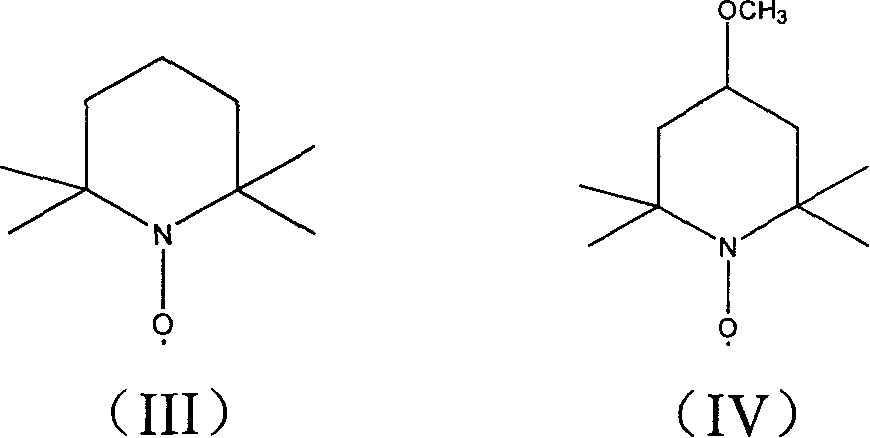
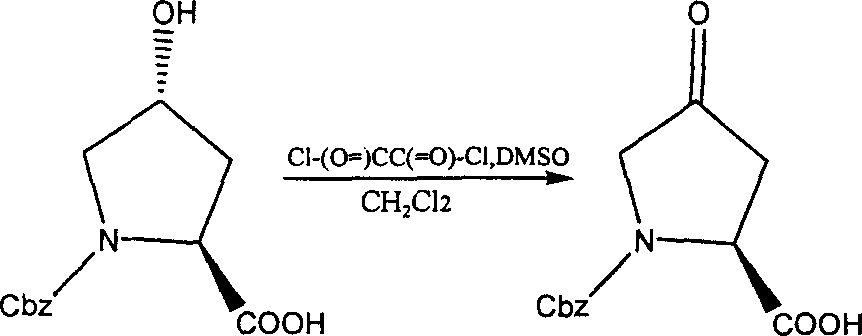Production of 4-carbonyl-(S)-proline derivative
A technology of proline and derivatives, which is applied in the field of synthesis of pharmaceutical intermediates 4-carbonyl-proline derivatives, can solve the problems of expensive process cost, high cost, harsh operating conditions, etc., and achieve good chemoselectivity , easy operation, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Will C 2 h 5 OCO-protected hydroxyproline 0.45g, 5% NaClO solution 5ml, 4-methoxy-2,2,6,6-tetramethyl nitroxide free radical piperidine 0.001g were added to 5ml solvent, at -25 ℃ React at -100°C for 5 minutes to 5 hours until the substrate is completely reacted, then remove excess oxidant with sodium bisulfite solution, wash and concentrate to obtain the oxidized product with a yield of 90%.
Embodiment 2
[0024] Add 0.50g of PNB-protected hydroxyproline, 6ml of 5% NaBrO solution, and 0.03g of 2,2,6,6-tetramethylnitroxyl piperidine (TEMPO) into 5ml of solvent, The reaction was carried out for 5 minutes to 50 minutes until the substrate was completely reacted, and then the excess oxidant was removed with sodium bisulfite solution, washed and concentrated to obtain the oxidized product with a yield of 88%.
Embodiment 3
[0026] Add 0.12 g of Cbz-protected hydroxyproline, 0.23 g of trichloroisocyanuric acid (TCCA), and 0.007 g of 2,2,6,6-tetramethylnitroxyl piperidine (TEMPO) into 2 ml of solvent, React at -25°C to 25°C for 15 minutes to 95 minutes until the substrate is completely reacted, then remove excess oxidant with sodium bisulfite solution, wash and concentrate to obtain the oxidized product with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com