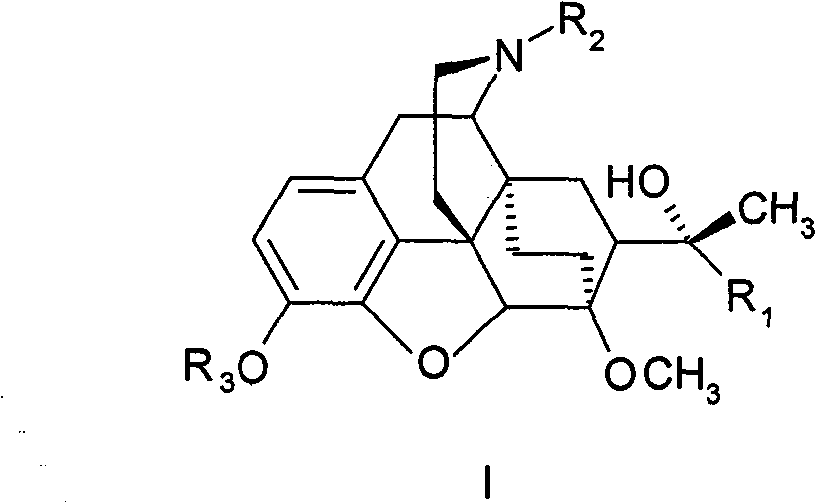
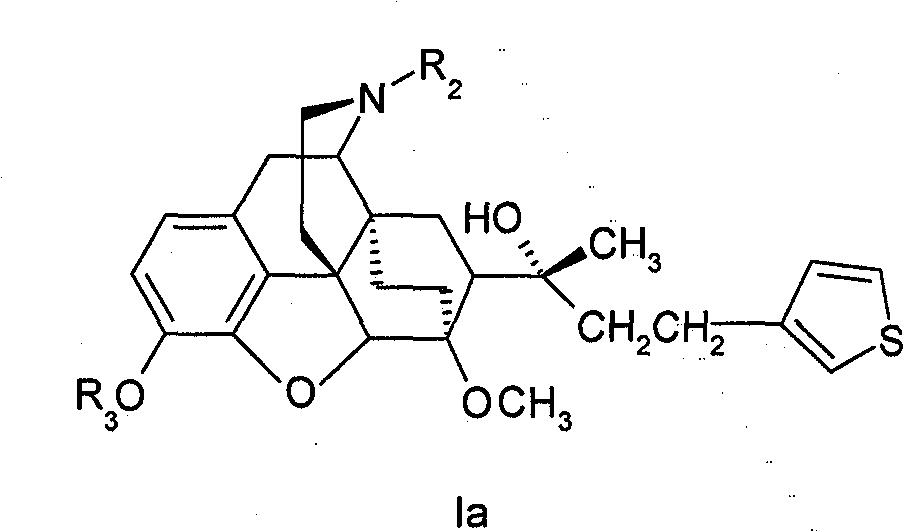
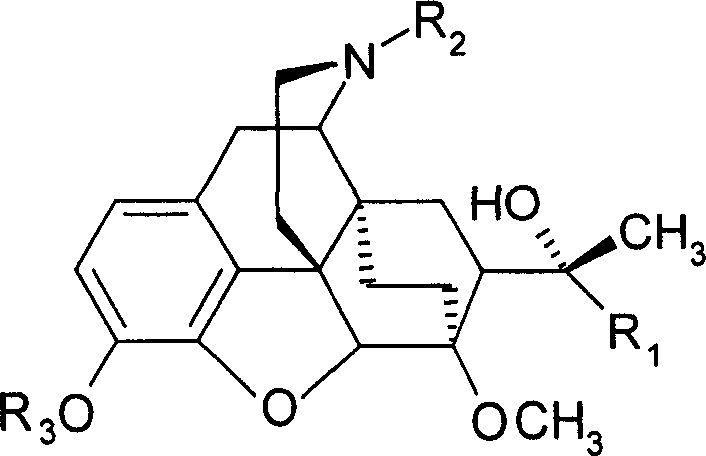
Eastern poppy base compound and its medical use
A technology of oripavine and compound, applied in the field of oripavine compound, can solve the problems of low analgesic efficacy, necessity of injection, low oral bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1. Preparation of 7α-[1-hydroxyl-1-methyl-3-(thiophen-3-yl)-propyl]-6,14-ethylene bridge tetrahydrooripavine (Ia-1)
[0075] 1.1 Preparation of 7α-vinyl-6,14-ethylene bridged tetrahydrothebaine (compound of formula III)
[0076] Put 100g of thebaine (compound of formula II) and 180ml of methyl ketene into the reaction flask, heat and reflux for 1 hour, distill off the remaining methyl ketene under reduced pressure, add 120ml of methanol, heat to dissolve, cool, and filter the solid. Washed twice with methanol and dried to obtain 112 g of the title compound (compound of formula III), melting point 118-120°C.
[0077] 1.2 Preparation of 7α-acetyl-6,14-ethyl bridged tetrahydrothebaine (compound of formula IV)
[0078] 40g of the compound of formula III obtained in Example 1.1, 8g of 10% palladium carbon and 400ml of absolute ethanol are placed in a hydrogenation kettle, and 40-50kg / cm2 of hydrogen gas is passed into it. 2 , carried out hydrogenation at 50-60°C fo...
Embodiment 2
[0085] Example 2. N-allyl-7α-[1-hydroxyl-1-methyl-3-(thiophen-3-yl)-propyl]-6,14-endoethylene bridged tetrahydronoropapium Preparation of Base (Ia-2)
[0086] 2.1 Preparation of N-cyano-7α-[1-hydroxy-1-methyl-3-(thiophen-3-yl)-propyl]-6,14-ethyl-tetrahydronorthebaine
[0087] Take 17.5 g of cyanogen bromide and dissolve it in 125 ml of chloroform, add 50.0 g of a solution of the compound of formula V dissolved in 275 ml of chloroform, reflux for 12 hours, evaporate the solvent after the reaction is completed, and treat with a small amount of absolute ethanol to obtain 48.0 g of white powder with a melting point of 198 -200°C. Elemental analysis (C 29 h 36 N 2 o 4 S): Calculated: C 68.75%, H 6.76%, N 5.53%; Found: C 68.84%, H 6.60%, N 5.49%.
[0088] 2.2 Preparation of 7α-[1-hydroxy-1-methyl-3-(thiophen-3-yl)-propyl]-6,14-endoethylenetetrahydronoripavine hydrochloride
[0089] Take 4g of the compound prepared in Example 2.1, 10g of potassium hydroxide and 50g of diethyle...
Embodiment 3
[0094] Example 3. N-Cyclopropylmethyl-7α-[1-Hydroxy-1-methyl-3-(thiophen-3-yl)-propyl]-6,14-endoethylene-bridged tetrahydronordong Preparation of Papaverine (Ia-3)
[0095] According to the method of Example 2.3, cyclopropylmethyl bromide was used instead of allyl bromide to obtain the title compound with a melting point of 178-180°C. 1 H-NMR (δppm): 9.05 (s, 1H); 7.42-7.04 (m, 3H); 6.71-6.53 (dd, 2H); 4.54 (s, 1H); 4.32 (s, 1H); 3H); 1.44(s, 3H).
[0096] According to the method of Example 1.5, the hydrochloride Ia-3·HCl of the above title compound was prepared. The melting point is 276-278°C. Elemental analysis (C 31 h 39 NO 4 S.HCl.1 / 2H 2 O): calculated: C 65.72%, H 7.24%, N 2.47%; found: C 65.35%, H 7.20%, N 2.32%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com