Chinese angelica root extract for treating cancer
An extract, angelica technology, applied in the field of angelica extract, can solve the problem of insufficient evidence and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] Preparation of Angelica Extract
[0023] Angelica sinensis (Angelicae sinensis; Dangqui) has long been used for blood disorders and female ailments. Generally, the dried root of Angelica esinensis (Oliv) Diels belonging to the family of Umbelliferae is used. Angelicae sinensis (hereinafter referred to as AS) is common knowledge in this technical field. Various techniques are known for extracting, isolating, and / or purifying individual active components of Angelicae sinensis. Organic solvent extracts of Angelicae sinensis can be obtained by any standard procedure customary in the art. According to the present invention, Angelicae sinensis is extracted with acetone, chloroform or hexanes. In a specific example of the present invention, the dried and powdered rhizomes of Angelicae sinensis are extracted using acetone as a solvent, and the obtained extract is AS-A. Furthermore, AS-A was further extracted with chloroform to obtain the extract AS-C; and AS-A was further e...
Embodiment 1
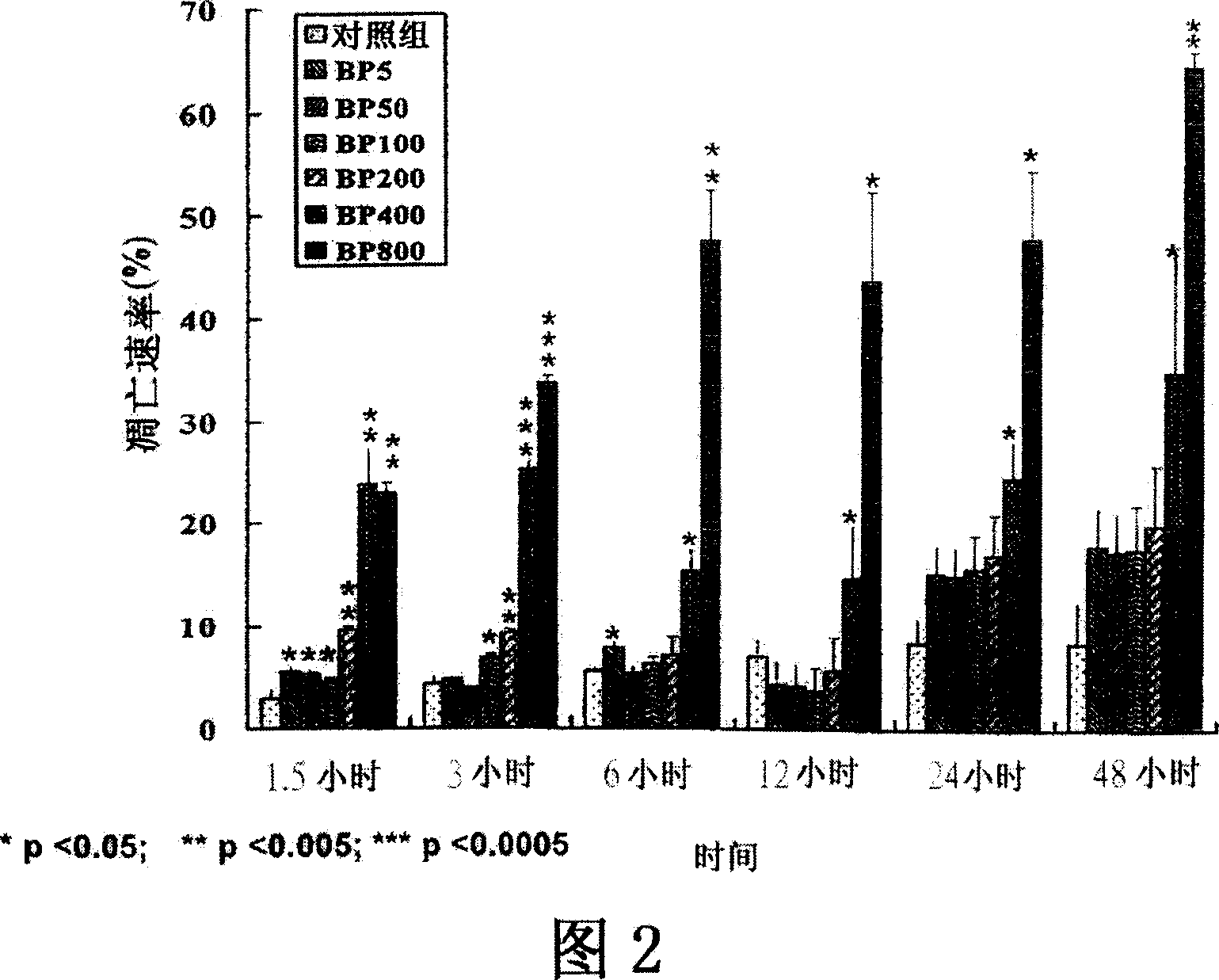
[0056] Example 1 - Analysis of Cytotoxicity
[0057] The effect on cell survival of treatments with different concentrations of Angelicae sinensis extract or its purified active ingredient was evaluated in triplicate MTT assays. Briefly, cells (5×10 3 ) into 96-well plates containing 100 μl of growth medium. After allowing the cells to attach for 24 hours, they were treated with 100 [mu]l of plant extracts or active ingredients dissolved in the culture medium. The control group contained 3 Exponentially growing cells were treated with different concentrations for 24, 48 and 72 hours. The cytotoxicity of each test substance is expressed as IC 50 A qualitative assay representing the concentration of drug required to cause 50% inhibition. All experiments in this study were repeated in triplicate.
[0058] Cytotoxicity (IC 50 )
normal cells
cancer cells
HUVECs
HDF
Caco-2
MCF-7
RG2
Extracts
(μg / ml)
AS-A
91...
Embodiment 2
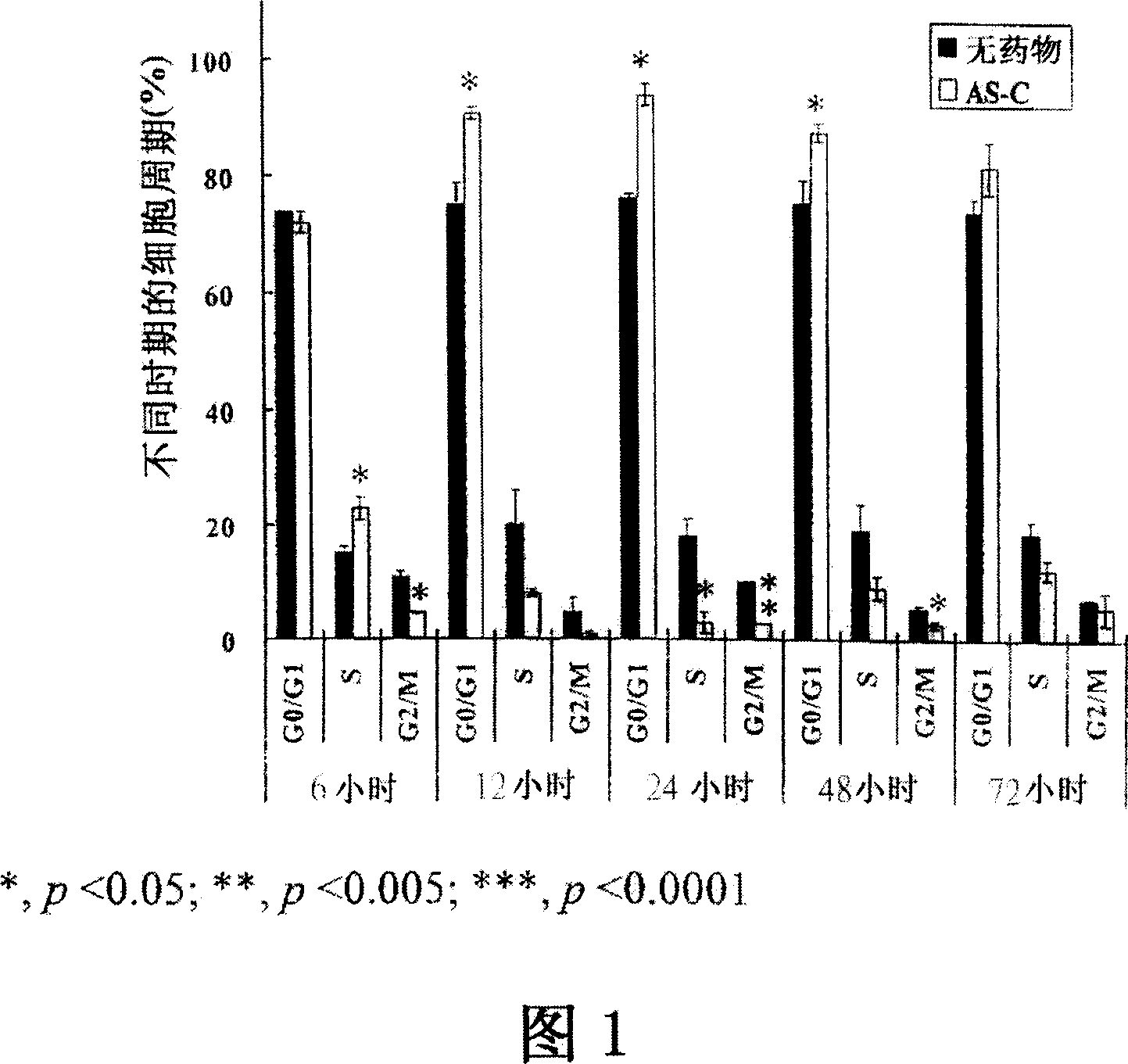
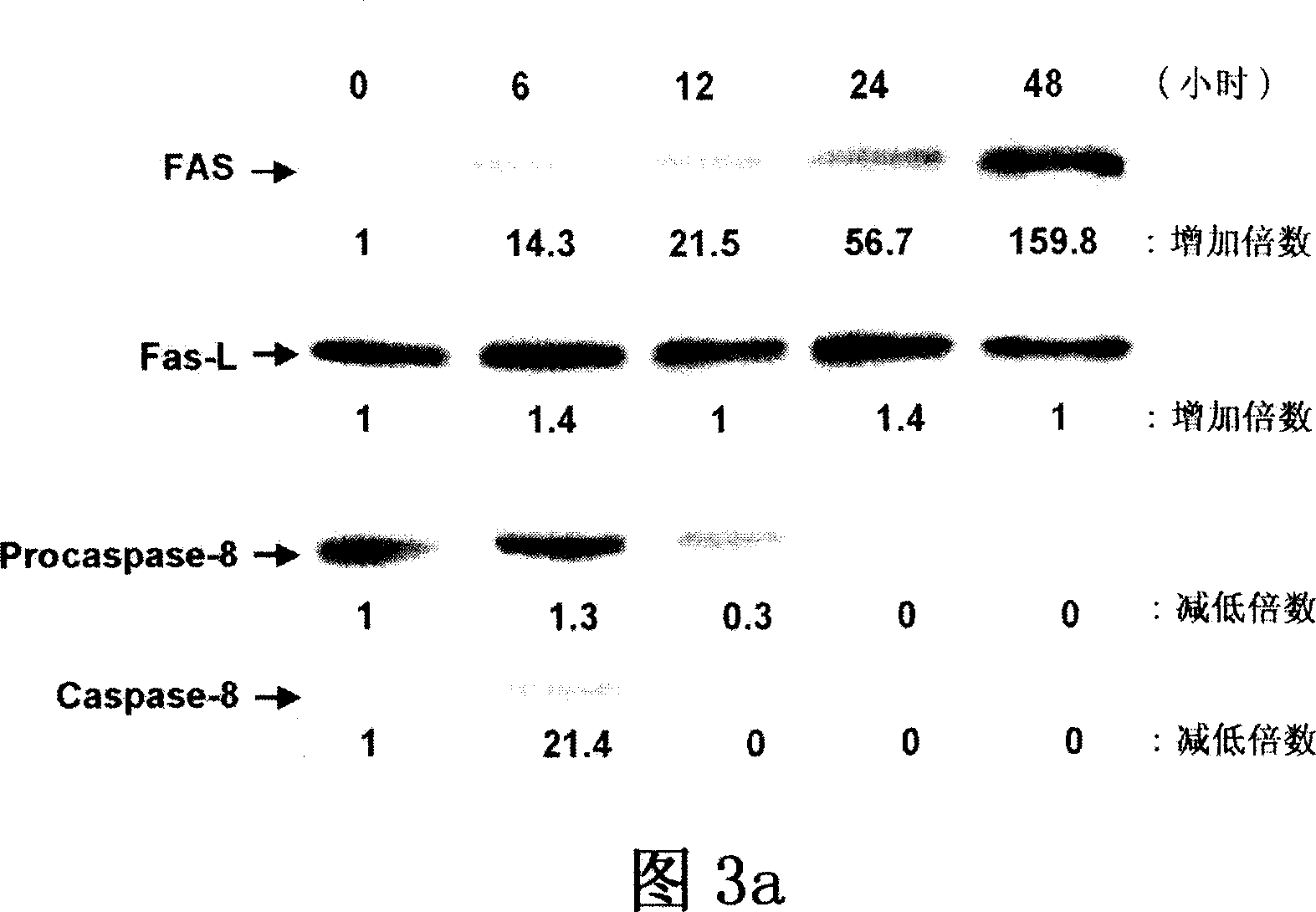
[0085] Example 2: AS-C and BP increase cell cycle arrest at G in GBM cells 0 / G 1 period
[0086] Brain tumor cell lines DBTRG-05MG and G5T / VGH were cultured in growth medium with diluent. In each test group and control group, DMSO was added and the content was less than 0.02% (v / v). In the treatment of AS-C and BP, 70 μg / ml of AS-C and 400 μM of BP were added, respectively. All samples were incubated for 48 hours. Analysis of cell cycle distribution was performed by staining DNA with propidium iodide (PI). In short, trypsin? Make 2×10 6 cell detachment. Detached cells and floating dead cells were centrifuged and washed twice with 10 ml of cold 1*PBS (Life Technologies). Aspirate the supernatant, suspend the cells in 0.8 ml of 1×PBS, add 200 μl of PI solution (50 μg / ml PI+0.05 mg / ml RNase A; Sigma Chemical Company), and refrigerate the cells overnight at 4°C. Cells were protected from light for at least 2 hours at room temperature prior to DNA analysis. After stainin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com