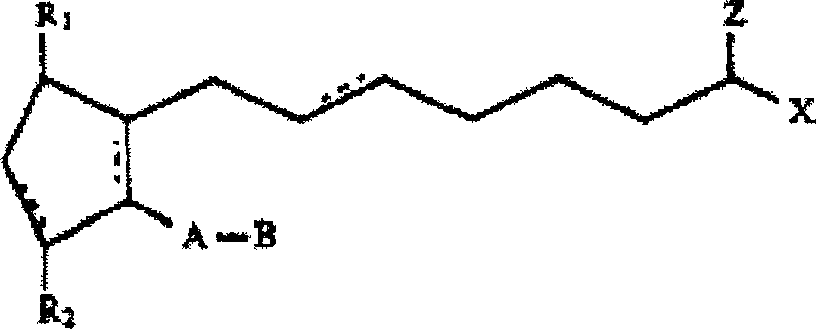
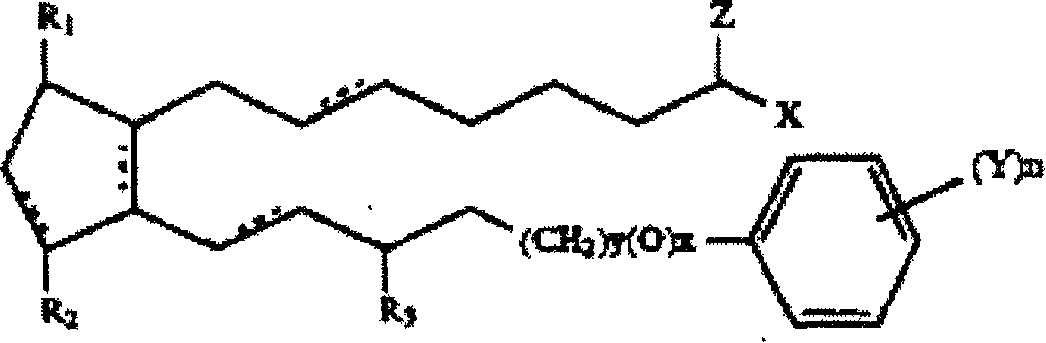
Biodegradable intraocular implants containing prostamides
A prostamide and biodegradable technology, applied in the field of intraocular implants and the preparation and use of the implants, can solve unwelcome problems and achieve the effect of prolonging the release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1 Preparation and testing of implants containing bimatoprost and a biodegradable polymer matrix
[0138] A biodegradable implant was prepared by combining bimatoprost and a biodegradable polymer composition. 800 mg polylactic acid (PLA) was combined with 200 mg bimatoprost. The composition was then dissolved in 25 ml of dichloromethane. The mixture was placed under vacuum at 45°C overnight to evaporate the dichloromethane. The resulting mixture was in the form of cast slabs. The cast plates were sheared and milled in a high shear mill with dry ice until the particles passed through a screen with a pore size of approximately 125 [mu]m. The microparticles were analyzed for the percentage of bimatoprost using high pressure liquid chromatography (HPLC). Dialysis was used to characterize the percent release of bimatoprost from the microparticles. The recovered granules were analyzed by HPLC for the percentage of residual bimatoprost.
[0139] Table 1 describes ...
Embodiment 2
[0142] Example 2 Production of Bimatoprost-Containing Biodegradable Intraocular Implants by Extrusion and Compression
[0143] The bimatoprost is combined with the biodegradable polymeric composition in a mortar and mortar. The composition was then mixed for about 15 minutes on a shaker set at about 96 RPM. Scrape the powdered mixture from the sides of the mortar and mix for an additional 15 minutes. The blended powder mixture was heated at the specified temperature for a total of 30 minutes to achieve a semi-molten state and form a polymer / drug melt.
[0144] Rods are manufactured as follows: Polymer / drug melt is granulated using 9-gauge polytetrafluoroethylene (PTFE) tubing, the granules are loaded into barrels and the material is extruded into filaments at a specific core extrusion temperature . The filaments are then cut into approximately 1 mg size implants or drug delivery systems. The stick size is approximately 2mm long x 0.72mm diameter. Rod implants weighed appr...
Embodiment 3
[0149] Example 3 Bimatoprost / PLA / PLGA intraocular implant for treating glaucoma
[0150] A 72 year old female with bilateral glaucoma was placed in each eye with an intraocular implant containing bimatoprost and a combination of PLA and PLGA. The implant weighs about 1 mg and contains about 500 mg bimatoprost. One implant is placed into the vitreous of each eye using a syringe. After about two days, the patient reported significant relief of eye discomfort. Examination indicated a decrease in intraocular pressure, with mean intraocular pressure falling from 28 mm Hg to 14.3 mm Hg measured at 8:00 AM. Patients were monitored monthly for about six months. Intraocular pressure levels below 15 mm Hg were maintained for six months, and the patient reported relief from ocular discomfort.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com