Bi-(4-hydroxybenzoyl) benzene monomer synthesis method
A technology of hydroxybenzoyl and hydroxybenzoyl, which is applied in the synthesis of semi-crystalline polyaryletherketone bis-benzene monomer and the field of toughness, which can solve the problem that the reaction product is prone to branching and affects the processing and performance of polymers, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
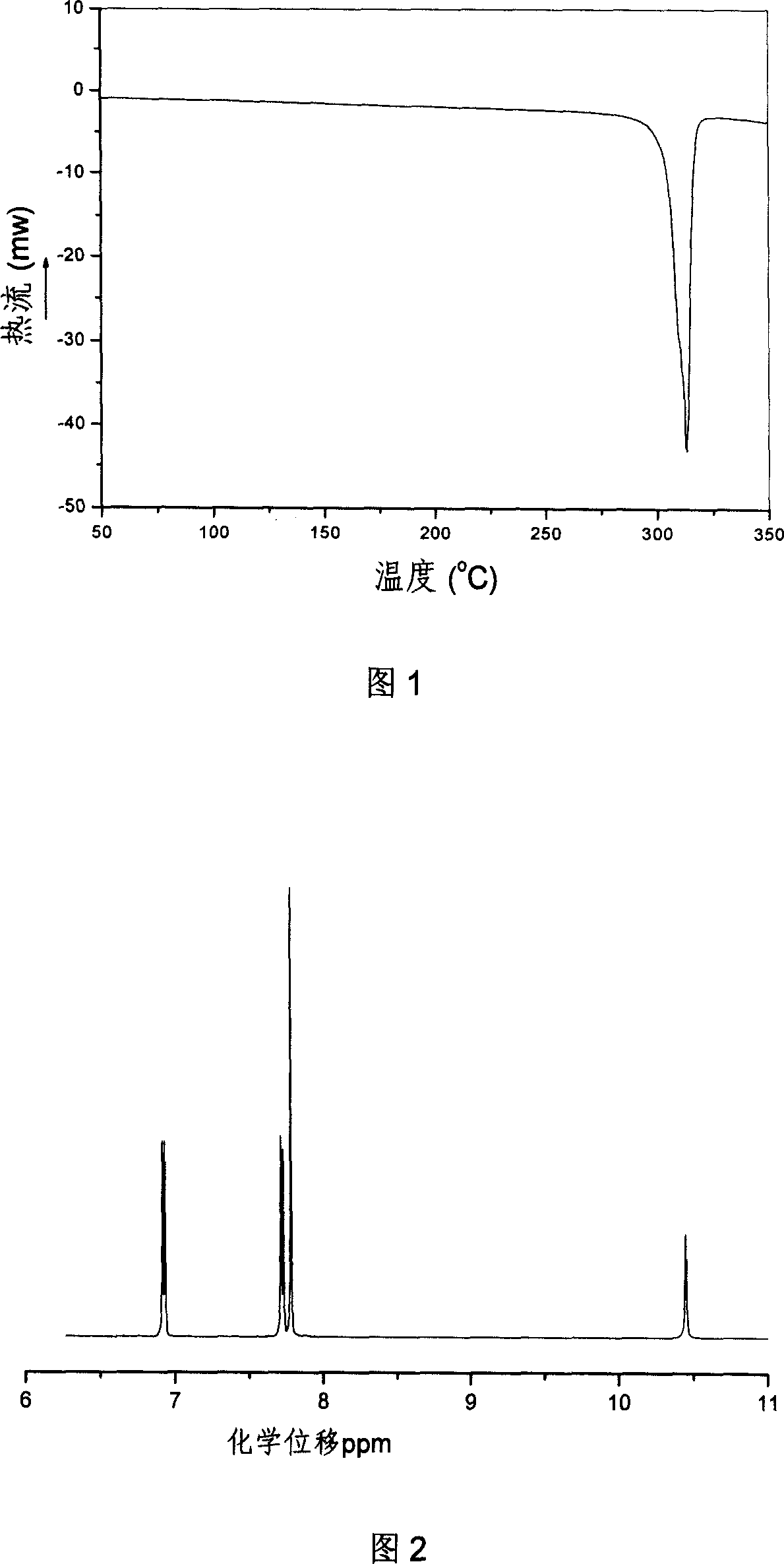
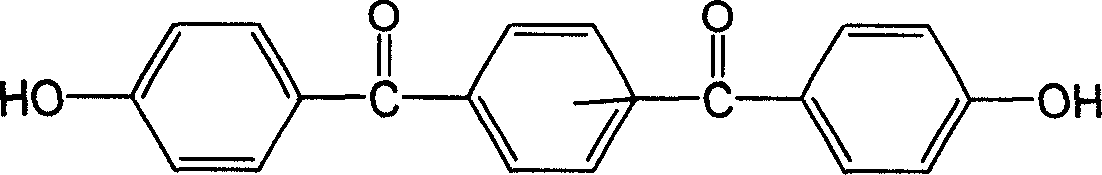
Image
Examples
Embodiment 1
[0023] Preparation of KOH solution: 280g (5mol) KOH was added to 500ml H 2 In a beaker or other container of O, dissolve to obtain an aqueous KOH solution; then 322g (1mol) two-1,4-(4-fluorobenzoyl)-benzene (purchased in the market) and the prepared KOH solution are added to the container Add 1000ml H 2 0 and 1500ml DMSO, stirred and heated to reflux, the stirring speed was controlled at 100-2000 rpm, the temperature of heating and reflux was 100 degrees, and the reflux temperature was maintained for 6h, the reaction solution was cooled to 25 degrees, and vacuum filtered through a Buchner funnel, The degree of vacuum is 18 mm Hg; slowly add concentrated hydrochloric acid to the filtrate, the concentration of concentrated hydrochloric acid is 36%, the rate of addition is controlled within 10ml / min, to ensure that the temperature of the system does not exceed 30 degrees, when the pH of the system reaches 11 When the hydrochloric acid was added, a white precipitate was obtained ...
Embodiment 2
[0026] The method is the same as example 1, 560gKOH is added to 1000mlH 2 O container was dissolved to obtain a KOH solution. Add this solution into the reaction vessel according to the method described in Example 1, stir and heat to reflux, keep the reflux temperature for 12 hours, cool the reaction solution and filter it; slowly add concentrated hydrochloric acid to the filtrate to PH=9, suction filter, A light yellow precipitate was obtained, which was repeatedly washed with water (or hot water) to pH = 7 and dried to obtain the monomer 4-hydroxybenzoyl-4'-fluorobenzophenone with a yield of 88%.
Embodiment 3
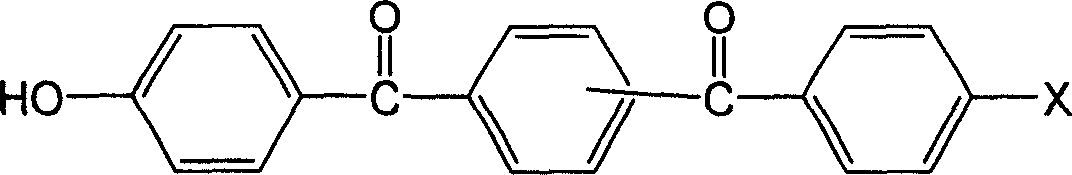
[0028] The method is the same as example 1, 560gKOH is added to 1000mlH 2 O container was dissolved to obtain a KOH solution. This solution was added to the reaction vessel according to the method described in Example 1, stirred and heated to reflux, and the reflux temperature was maintained for 8 hours, and the reaction solution was cooled and filtered; hydrochloric acid was slowly added to the filtrate to adjust the pH to 7, and suction filtered. Obtain white (or pale yellow precipitate), wash the precipitate repeatedly with water (or use hot water) to PH=7, dry to obtain monomer bis-1,4-(4-hydroxybenzoyl)-benzene with a yield of 85 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com