Lyophilized powder injection of breviscapinum and preparation process thereof
A technology for freeze-dried powder injection and breviscapine, which is applied to breviscapine for injection, scutellarin for injection, mannitol and sodium bicarbonate, and the field of breviscapine, which can solve the problem of poor formability of breviscapine and breviscapine. Problems such as poor solubility and difficulty in prescription research can achieve the effect of improving and treating blood circulatory system diseases, with remarkable efficacy and stable and reliable products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
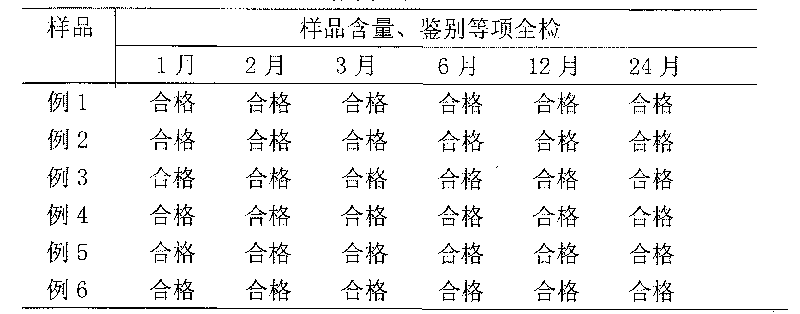
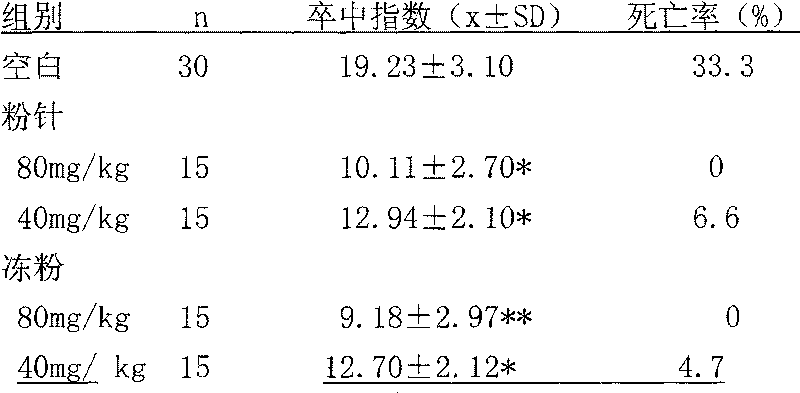
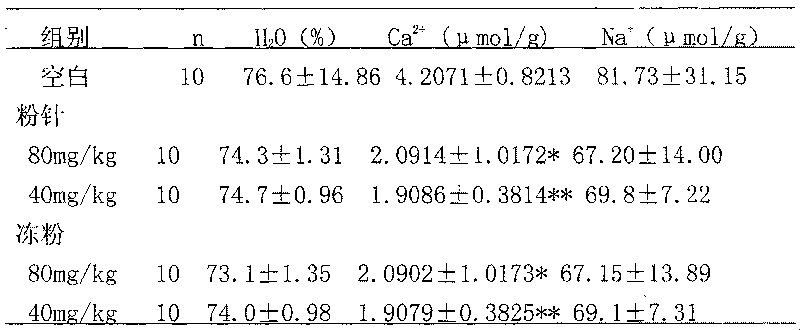
Examples
Embodiment 1
[0026] Prescription (1000 sticks)
[0027] Breviscapine 50g(50.25%)
[0028] Mannitol 40g (40.20%)
[0029] Sodium bicarbonate 9.5g (9.55%)
[0030] Product specification: 50mg / bottle
[0031] Process: Under the condition of 10,000 grades, take the above-mentioned qualified breviscapine raw materials for injection (self-inspection increases heat source, abnormal toxicity, hemolysis, etc.) and auxiliary materials and dissolve them in 1800ml of water for injection. After decolorization, add Dilute with water for injection to a total volume of 2000ml. First filter with fast filter paper, then filter the filtrate through filter paper and 0.45μm filter membrane, and then filter it with a filter membrane below 0.2μm under the condition of 100 grades. Cover with a slotted lid, send it to a freezer vacuum dryer, quickly cool to -35°C ~ -40°C, take 2 to 3 hours, gradually heat up to 35°C ~ 45°C, tighten the lid, take out the product, cover and pack , Inspection, and qualified medi...
Embodiment 2
[0034] Prescription (1000 sticks)
[0035] Breviscapine 50g(47.17%)
[0036] Mannitol 46g (43.40%)
[0037] Sodium bicarbonate 10g (9.43%)
[0038] Product specification: 50mg / bottle
[0039] Technology: technology requirement and process are basically the same as embodiment 1.
Embodiment 3
[0041] Prescription (1000 sticks)
[0042] Breviscapine 55g (47.21%)
[0043] Mannitol 50g (42.92%)
[0044] Sodium bicarbonate 11.5g (9.87%)
[0045] Product specification: 50mg / bottle
[0046] Technology: technology requirement and technological process are basically the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com