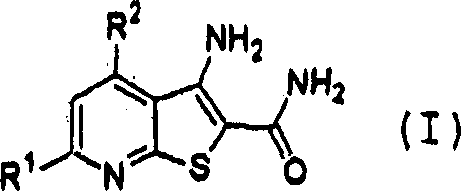
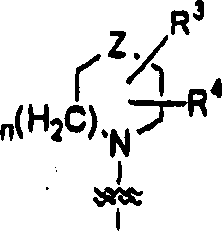
Thienopyridine derivative
A compound and substituent technology, applied in the field of compounds that promote bone formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0329] (Example 1) 3-Amino-4-(dimethylamino)thieno[2,3-b]pyridine-2-carboxamide (exemplified compound No. 2-17)
[0330] With reference to the method of Pharm. Chem. J. (Engl. Transl.), 26, (1992), 870-874, it was prepared as follows.
[0331] (1a)(2Z)-2-cyano-3-(dimethylamino)but-2-enethioamide
[0332] 1.00 g (10 mmol) of cyanothioacetamide and 1.73 g (13 mmol) of N,N-dimethylacetamide dimethyl acetal were dissolved in 5 mL of acetonitrile, and stirred at room temperature for 1 hour. The precipitated crystals were collected by filtration, and the crystals were washed with acetonitrile to obtain 1.05 g of the title compound (yield 62%).
[0333] Mp 155-158℃;
[0334] 1 H NMR(DMSO-d 6 , 400MHz) δ 2.27 (3H, s), 3.03 (6H, s), 8.08 (1H, br), 8.83 (1H, br).
[0335] (1b) 4-(Dimethylamino)-2-thio-1,2-dihydropyridine-3-carbonitrile
[0336] 1.05g (6.2mmol) of (2Z)-2-cyano-3-(dimethylamino)but-2-enethioamide prepared in Example 1(1a) and 2.22g (18.6mmol) of N, The N-dimethylformamide dim...
Embodiment 2
[0346] (Example 2) 3-Amino-4-(diethylamino)thieno[2,3-b]pyridine-2-carboxamide (exemplified compound No. 2-33)
[0347] (2a)(2Z)-2-cyano-3-(diethylamino)but-2-enethioamide
[0348] 406 mg (2.38 mmol) (2Z)-2-cyano-3-ethoxybut-2-ene thioamide (J. Org. Chem., (1962), 27, 2433-2439) and 0.36 mL ( 3.53 mmol) diethylamine was suspended in 5 mL of ethanol and stirred at room temperature for 2 hours. The solvent was distilled off, and the resulting residue was purified by silica gel column chromatography (ethyl acetate / hexane=2:1) to obtain 237 mg of the title compound (yield 50%).
[0349] 1 H NMR(CDCl 3 , 400MHz) δ1.32 (6H, t, J = 7.04 Hz), 2.71 (3H, s), 3.65 (4H, q, J = 7.05 Hz), 6.69 (2H; br s).
[0350] (2b) 4-(Diethylamino)-2-thio-1,2-dihydropyridine-3-carbonitrile
[0351] (2Z)-2-cyano-3-(diethylamino)but-2-enethioamide prepared in Example 2(2a) was used instead of (2Z)-2-cyano-3-(dimethyl (Ylamino)but-2-enethioamide was reacted in the same manner as in Example 1(1b) to obtain th...
Embodiment 3
[0360] (Example 3) 3-Amino-4-(dimethylamino)-6-methylthieno[2,3-b]pyridine-2-carboxamide (exemplified compound No. 2-102)
[0361] (3a) 4-(Dimethylamino)-6-methyl-2-thio-1,2-dihydropyridine-3-carbonitrile
[0362]1.46 g (7.5 mmol) 2-chloro-4-(dimethylamino)-6-methylnicotinonitrile (Pharm.Chem.J., (Engl.Transl.), 25, (1991), 623-628 .) and 0.74 g (9.7 mmol) of thiourea were suspended in 25 mL of toluene, and stirred under heating and reflux for 4 hours. 40 mL of ethanol was added to the reaction mixture, and then heated to reflux for 30 minutes. Leave it at room temperature overnight, filter the precipitated solid, and wash with ethanol, water, and ethanol in sequence to obtain 0.64 g of the crude product of the title compound.
[0363] 1 H NMR(DMSO-d 6 , 400MHz) δ 2.20 (3H, s), 3.18 (6H, s), 6.23 (1H, s), 12.41 (1H, br).
[0364] (3b) 3-Amino-4-(dimethylamino)-6-methylthieno[2,3-b]pyridine-2-carboxamide
[0365] 0.19g (1.0mmol) of 4-(dimethylamino)-6-methyl-2-thio-1,2-dihydropyrid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com