Method for producing polyhalogenated diamantane and derivative thereof
A technology of adamantinetetradecane and polyhalogenated adamantine, applied in the field of manufacturing polyhalogenated adamantinetetradecanes and adamantinetetradecane polyols, can solve the problem of low reactivity and lack of adamantinetetradecane Alcohols, low reaction yield and other problems, to achieve the effect of low reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Under nitrogen flow, add 40 g of concentrated sulfuric acid (2 times the mass of adamantine tetradecane) and 1.5 g of sodium sulfate (0.0106 mol, 0.1 times the mole of adamantine tetradecane) into a 500 ml four-neck flask. Then 20 g (0.106 mol) of adamantine tetradecane was added, and stirred for 10 minutes while maintaining the suspended state at about 20°C. Then, 24.7 g (0.212 mol, 2 times mol of adamantinetetradecane) of chlorosulfonic acid was added slowly so that the reaction would not run out, and it stirred at 30 degreeC for 3 hours. The reaction solution after 3 hours was in a suspended state. As a result of analyzing the reaction solution by GC, it was found that the adamantinetetradecane used as raw materials was 74% by mass, the monochloroadamantatetradecane was 2% by mass, the dichloroadamantotetradecane was 22% by mass, and the trichloroadamantotetradecane was 2% by mass, the progress of the reaction came to a halt. Then, drop into chlorosulfonic acid 37....
Embodiment 2~4
[0143] Except that the amount of chlorosulfonic acid used in Example 1 was changed according to Table 1, the same operation was carried out. The results are shown in Table 1.
[0144] Table 1
[0145] Chlorosulfonic acid (molar multiple)
Embodiment 5
[0147] Operate and react in the same manner as in Example 1, and carry out aftertreatment. To the obtained crude product, 20 g of n-heptane (one time the mass of the raw material adamantinetetradecane) was added, followed by heating and reflux for 2 hours. Liquids are often in suspension. Then, it was cooled to 5° C., stirred for 5 hours and matured, and the solid was filtered to obtain 22 g (containing 96% of dichloroadamantyl tetradecane) of a white solid, with a yield of 78% (based on adamantine tetradecane).
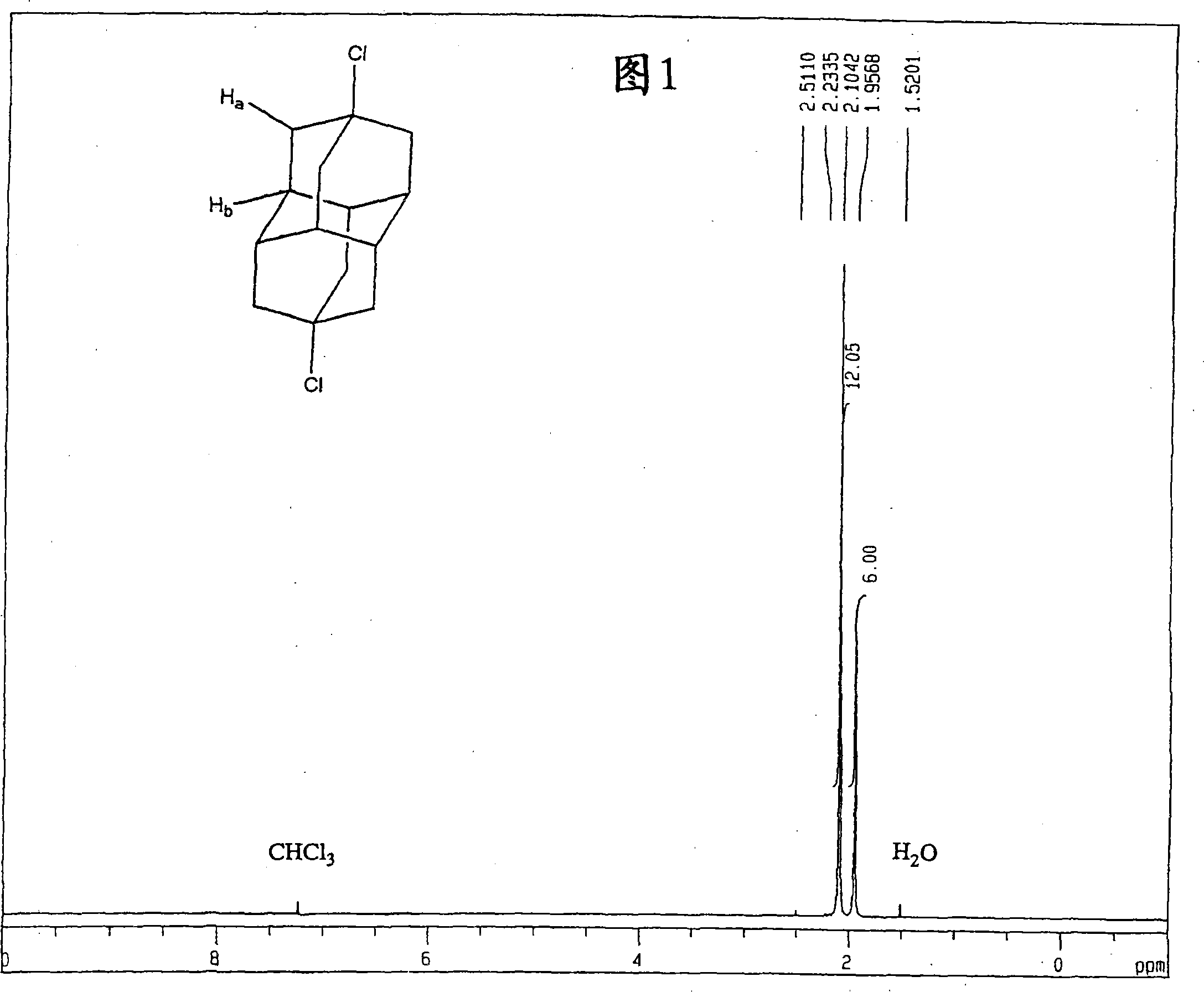
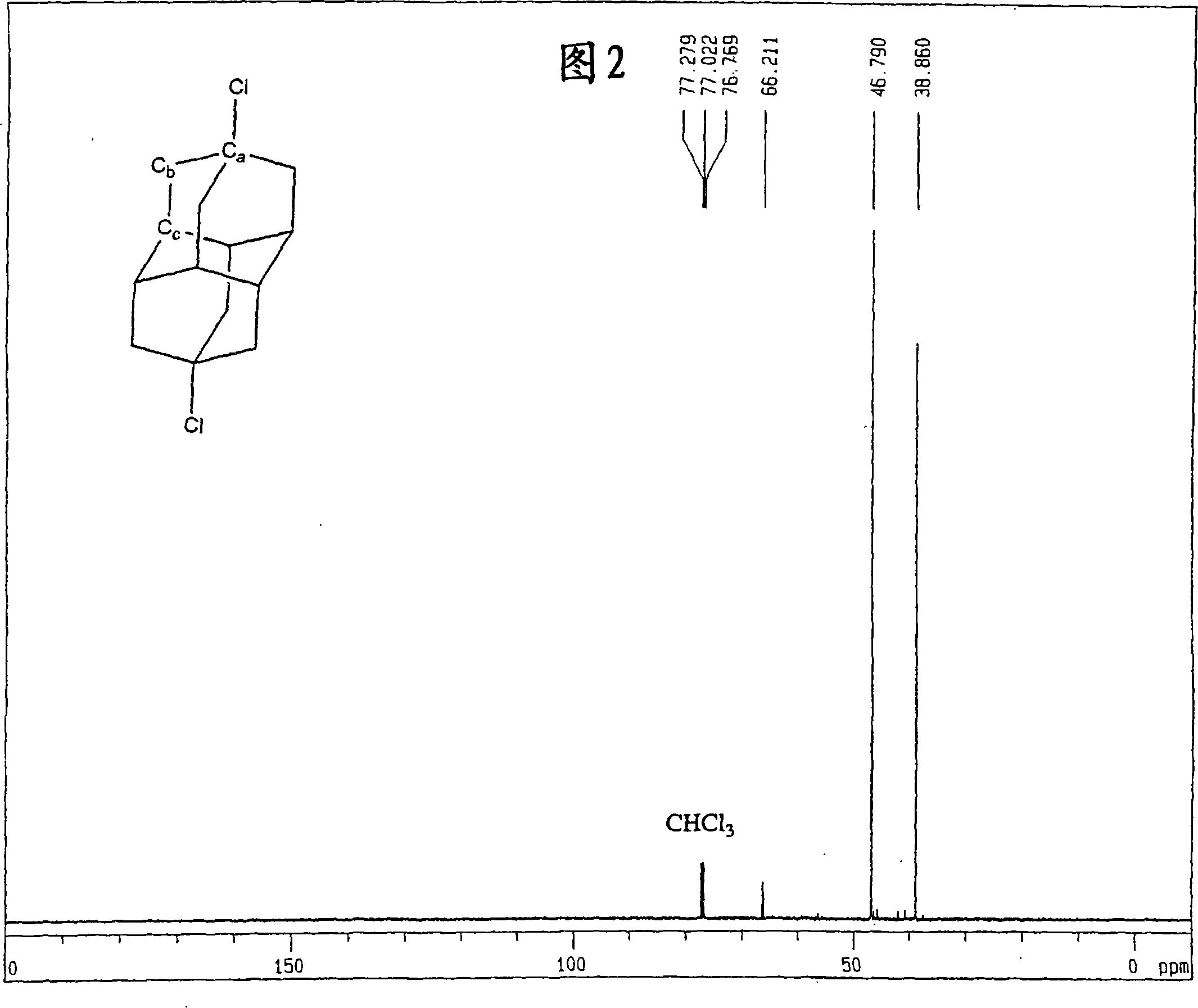
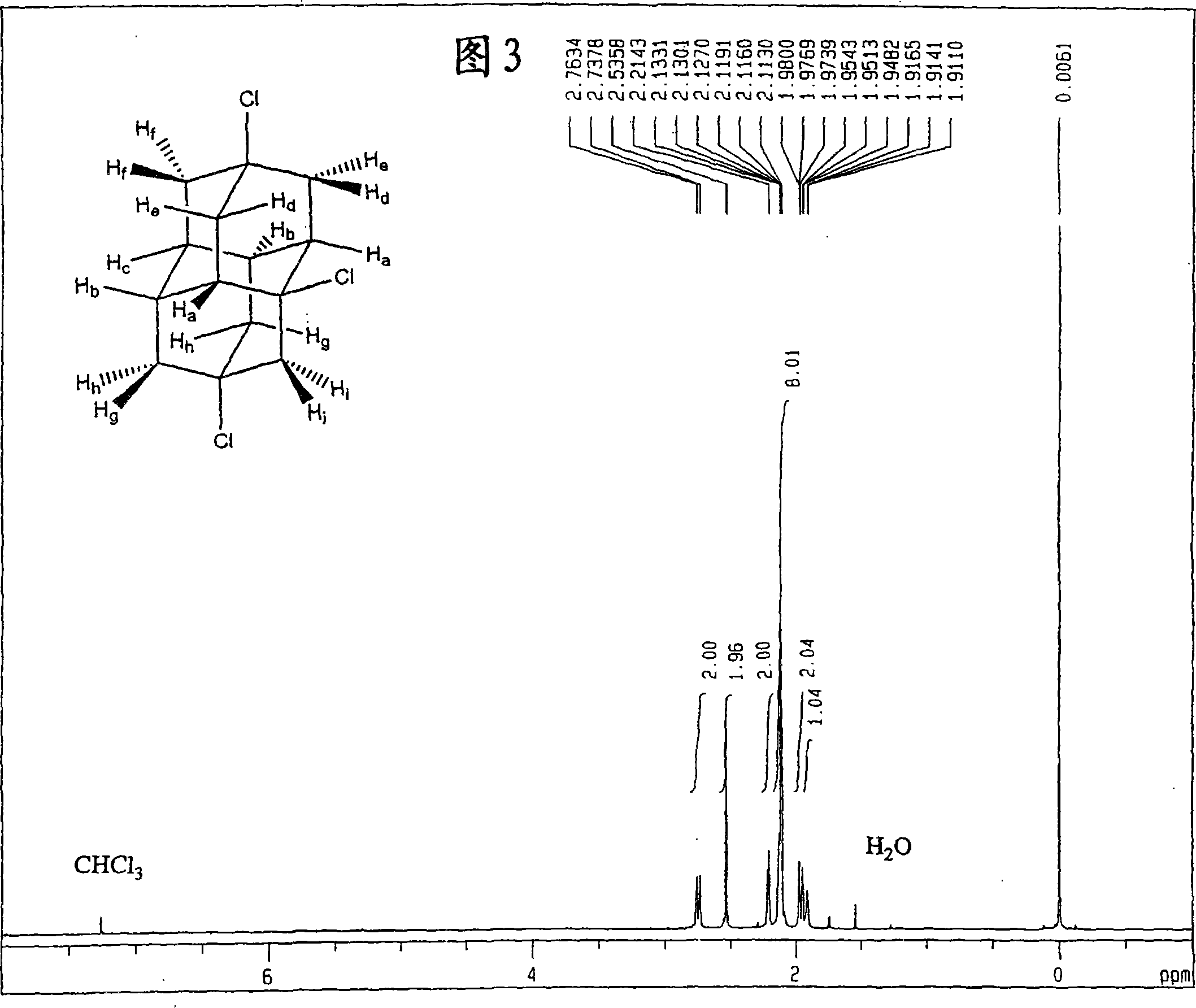
[0148] The obtained dichloroadamantotetradecane is almost selectively 4,9-dichloroadamantotetradecane. FIG. 1 shows a nuclear magnetic resonance (NMR) spectrum of protons, and FIG. 2 shows a nuclear magnetic resonance (NMR) spectrum of carbon.
[0149] MASS (EI): molecular weight 256 (M + )
[0150] 1 H-NMR spectrum (TMS reference): δ2.10 (H a , s, 12H), δ1.96(H b , s, 6H)
[0151] 13 C-NMR spectrum (TMS reference): δ66.2 (C a ), δ46.7(C b ), δ38.9(C c ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com