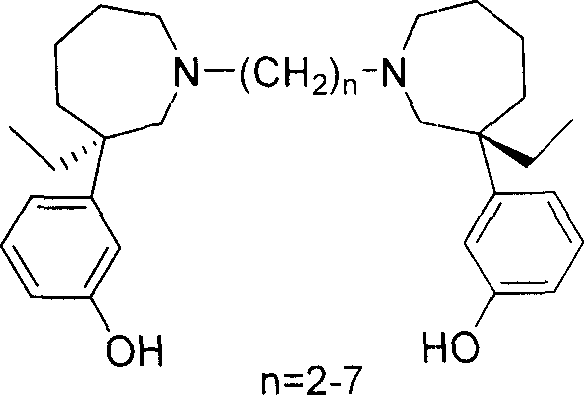
(+)-Meptazinol diligand derivative and/or its salt and the prepn process
A technology of meptanol and methyl meptanol, which is applied in the directions of drug combinations, active ingredients of heterocyclic compounds, nervous system diseases, etc., can solve the problems such as the lack of dual ligand derivatives.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of (+)-N-desmethylmeprotamol
[0027] (+)-Mebutamol (11.15g, 47.85mmol) was added to 700ml of chloroform, KHCO 3 (83.6g, 836mmol) and ethyl chloroformate (35ml, 347.9mmol). The reaction was refluxed for 2 hours. After the reaction solution was cooled, 500ml of water was added to make the KHCO 3 dissolved, the chloroform layer was separated, dried and concentrated, and the obtained yellow oil was dissolved in 830ml of methanol, and K 2 CO 3 (76.6g, 555mmol) dissolved in 830ml of water. React at room temperature for 1 hour. The methanol was distilled off, the pH was adjusted to ≈5 with hydrochloric acid, and extracted with 250ml×3 ether. The ether layers were combined, and the obtained oil (+)-N-ethoxycarbonyl-N-desmethylmeprotamol was added to 120 ml of 50% sulfuric acid and refluxed for 4 hours. Stop the reaction, cool in an ice-salt bath, add 150ml of chloroform, adjust the pH to 9 with ammonia water, and separate the liquids. The aqueous layer was ...
Embodiment 2
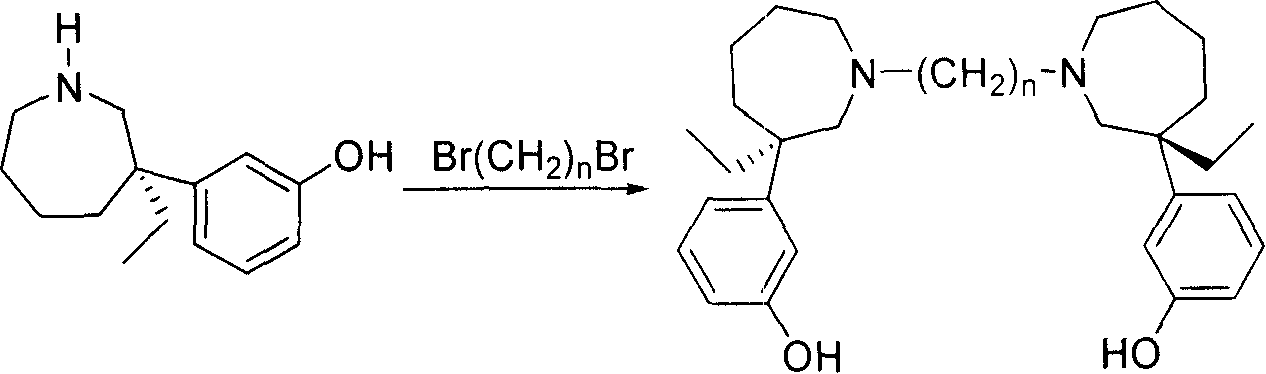
[0031] Preparation of N, N'-(1,2-ethyl)-bis-(+)-normaprotamol hydrochloride
[0032] (+)-N-desmethylmaprotamol (0.75g, 3.42mmol) was added to 6ml of acetonitrile, triethylamine (950μL, 6.84mmol), and 1,2-dibromoethane (148μL, 1.71mmol), The reaction was refluxed for 3 hours. After cooling, the reaction solution was concentrated to obtain 1.16 g of light yellow foam, and silica gel column chromatography obtained 0.55 g of N,N'-(1,2-ethyl)-bis-(+)-normaprotamol, with a yield of 69%.
[0033] N,N'-(1,2-ethyl)-bis-(+)-normaprotamol (0.55g, 1.19mmol) was dissolved in 10ml of anhydrous ether. Anhydrous HCl-ether solution was added dropwise to adjust the pH to ≈3, and a large number of light yellow solids were precipitated. Filtration, infrared drying to obtain N,N'-(1,2-ethyl)-bis-(+)-normeprotamol hydrochloride 0.58g, mp.156-160℃, [α] D =+23.29° (c=0.108, MeOH).
[0034] 1 HNMR (CDCl 3 ): δ7.19-7.15 (t, 1H, Ar-H, J=8), 6.89-6.82 (m, 2H, Ar-H), 6.72-6.69 (m, 1H, Ar-H), 6.4 (br...
Embodiment 3
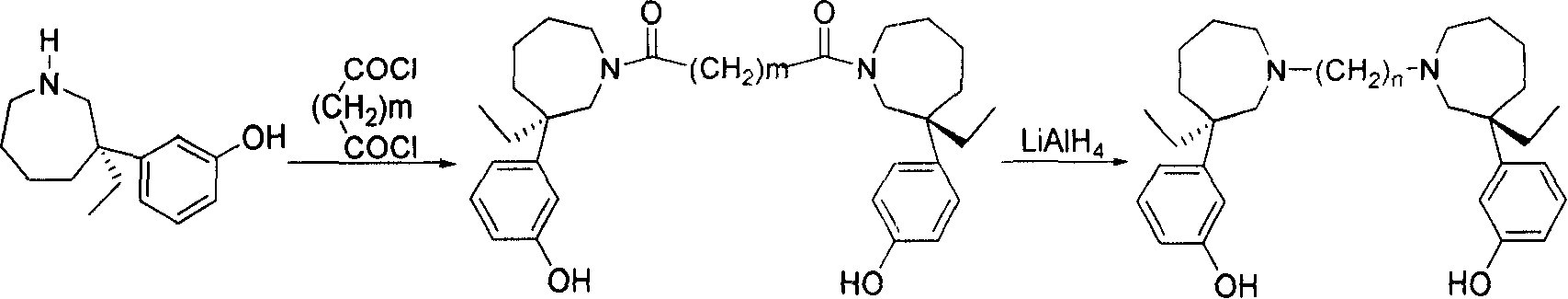
[0037] Preparation of N, N'-(1,4-butyl)-bis-(+)-normaprotamol hydrochloride
[0038] Add (+)-N-desmethylmaprotamol (1.57g, 7.17mmol) to 15ml of anhydrous dichloromethane, cool in an ice-water bath, and add triethylamine (2ml, 14.38mmol). Succinoyl chloride (410 μL, 3.55 mmol) was diluted with 3 ml of anhydrous dichloromethane, and added dropwise to the reaction solution. The reaction stopped after 10 minutes. The reaction solution was washed with 2ml of water and 3ml of 1N HCl. The organic layer was dried. Filter and distill off the solvent to obtain a brown foam. Silica gel column chromatography yielded 1.35 g of N,N'-(1,4-succinoyl)-bis-(+)-N-desmethylmeprotamol with a yield of 73%.
[0039] LiAlH 4(0.52g, 13.68mmol) and 20ml of anhydrous tetrahydrofuran, stirred at room temperature. Dissolve N,N'-(1,4-succinoyl)-bis-(+)-N-desmethylmeprotamol (1.35g, 2.55mmol) in 35ml of anhydrous THF and add dropwise to the reaction solution . After the dropwise addition was complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com