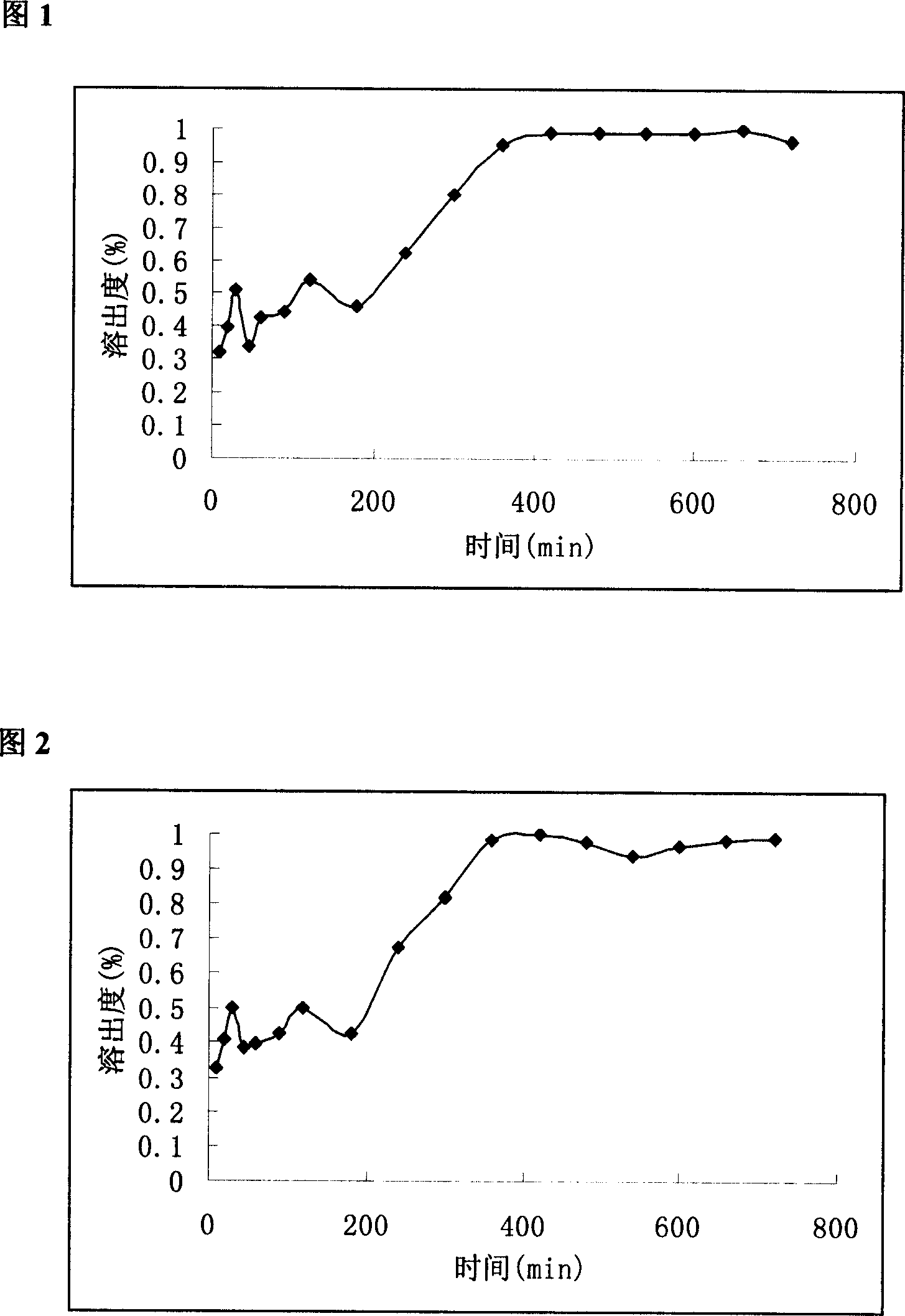
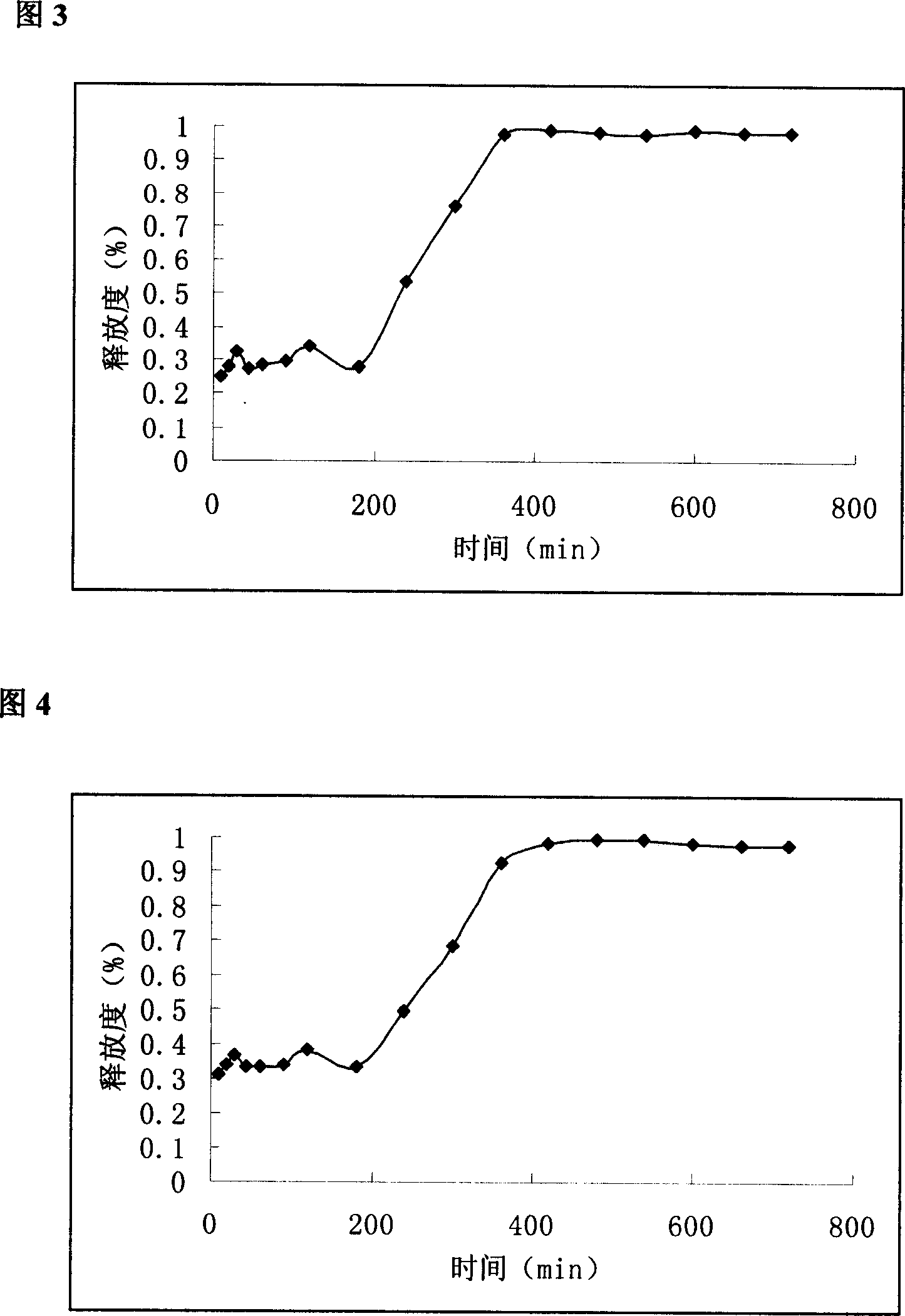
Slow control released methylphenidate hydrochloride capsule and its preparing method
A technology of methylphenidate hydrochloride and capsules, which is applied in the field of sustained and controlled release pharmaceutical preparations, sustained and controlled release capsule preparations and their preparation, can solve the problems of obvious side effects, short half-life, poor compliance, etc. The effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prepare drug-loaded pellets according to the following composition ratios
[0036] Blank ball core 100g
[0037] Methylphenidate Hydrochloride 30g
[0038] Polyvinylpyrrolidone 3g
[0039] Polyethylene glycol-4000 0.3g
[0040] Prepare quick-acting pellets according to the following composition ratio
[0041] Drug-loaded pellets 100g
[0042] Hypromellose 2.5g
[0043] Polyethylene glycol-4000 0.5g
[0044] Prepare medium-effect pellets according to the following composition ratio
[0045] Drug-loaded pellets 100g
[0046] Surelease® Solids 6g
[0047] Prepare long-acting pellets according to the following composition ratio
[0048] Drug-loaded pellets 100g
[0049] Surelease® solid content 12g
[0050]Methylphenidate hydrochloride is micronized and dissolved in polyvinylpyrrolidone aqueous solution, polyethylene glycol-4000 is added under stirring, and a coating solution is obtained after ultrasonication. The blank ball core is coated with a fluidized bed, ...
Embodiment 2
[0055] Prepare drug-loaded pellets according to the following composition ratios
[0056] Blank ball core 100g
[0057] Methylphenidate Hydrochloride 10g
[0058] Polyvinylpyrrolidone 3g
[0059] Polyethylene glycol-6000 small amount
[0060] Prepare quick-acting pellets according to the following composition ratio
[0061] Drug-loaded pellets 100g
[0062] Methylcellulose 2g
[0063] Talc Trace
[0064] Prepare medium-effect pellets according to the following composition ratio
[0065] Drug-loaded pellets 100g
[0066] Cellulose acetate 3g
[0067] Triethyl citrate 0.15g
[0068] Prepare long-acting pellets according to the following composition ratio
[0069] Drug-loaded pellets 100g
[0070] Cellulose acetate 5g
[0071] Triethyl citrate 0.25g
[0072] The preparation process was the same as in Example 1. Take 80 g of quick-acting pellets, 100 g of medium-acting pellets, and 80 g of long-acting pellets, mix them uniformly, and pack them into capsule shells at a...
Embodiment 3
[0074] Prepare drug-loaded pellets according to the following composition ratios
[0075] Blank ball core 100g
[0076] Methylphenidate Hydrochloride 80g
[0077] Hypromellose 3.5g
[0078] Glyceryl triacetate small amount
[0079] Prepare quick-acting pellets according to the following composition ratio
[0080] Drug-loaded pellets 100g
[0081] Polyvinylpyrrolidone 2g
[0083] Prepare medium-effect pellets according to the following composition ratio
[0084] Drug-loaded pellets 100g
[0085] Eudragit E30D 6g
[0086] Triethyl citrate 1.5g
[0088] Prepare long-acting pellets according to the following composition ratio
[0089] Drug-loaded pellets 100g
[0090] Eudragit E30D 9g
[0091] Triethyl citrate 2.5g
[0092] Talc powder 4g
[0093] The preparation process was the same as in Example 1. Take 20 g of quick-acting pellets, 80 g of medium-acting pellets, and 50 g of long-acting pellets, mix them uniformly, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com