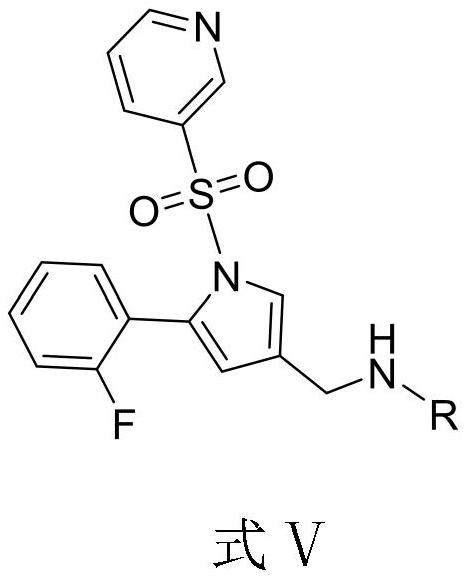
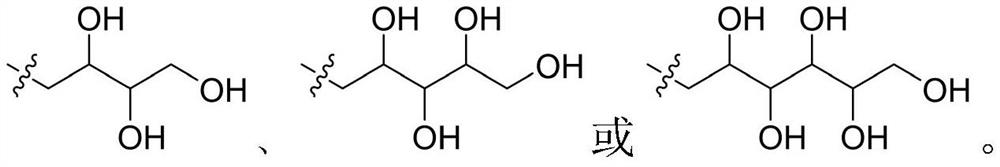
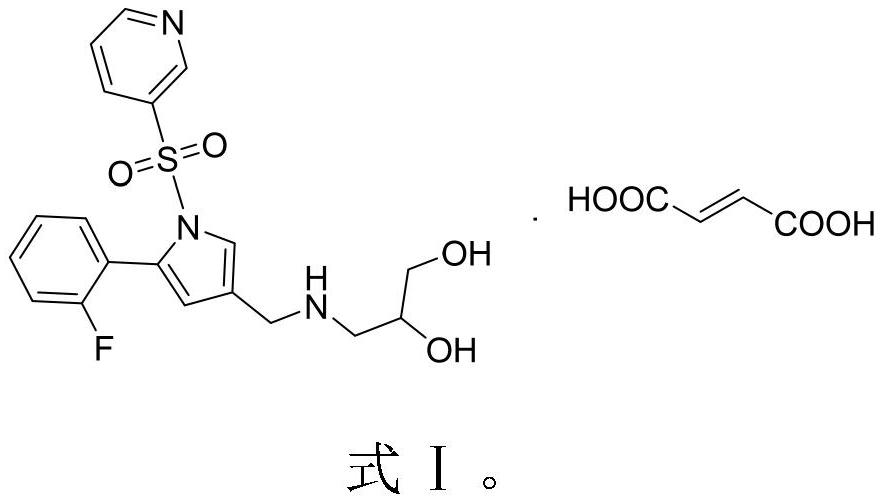
Compound for treating peptic ulcer and preparation method and application thereof
A compound and chemical structural formula technology, applied in the field of medicine, can solve problems such as poor water solubility of fumaric acid vonoprazan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde (compound IV)
[0030]
[0031] At room temperature, add 30 g of 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde (compound II), 100 ml of acetonitrile (CHCN), 29 g of N,N-diisopropylethyl Amine (DIEA) and 4g of 4-dimethylaminopyridine (DMAP), add 35g of pyridine-3-sulfonyl chloride (compound III) at room temperature, stir and react at room temperature for 30min, after the reaction is complete, add water to quench the reaction, use Adjust the pH to 5 with hydrochloric acid, cool down to room temperature, stir and crystallize, filter, and dry under reduced pressure to obtain compound (IV), which is 44 g of yellow powder with a yield of 84%.
[0032] 1H-NMR (CDCl 3 )δ: 9.90 (1H, s), 8.81 (1H, dd, J = 1.4, 4.8Hz), 8.59 (1H, d, J = 2.2Hz), 8.15 (1H, d, J = 1.8Hz), 7.71 ( 1H,dt,J=1.9,8.2Hz), 7.45(1H,m), 7.37(1H,dd,J=4.9,8.1Hz), 7.16(2H,m), δ7.01(1H,t,J= 8.9Hz), 6.67 (1H,d,J=1.7Hz). ...
Embodiment 2
[0033] Example 2 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-propylene glycol methylamine monofumaric acid (compound Ⅰ) synthesis
[0034]
[0035] (1) At room temperature, add 43g of compound (IV) and 200ml of methanol to the reaction flask, add 15g of 3-amino-1,2-propanediol, stir for 0.5h, then add 2g of sodium borohydride in N, N-dimethylacetamide solution, stirred and reacted at room temperature for 0.5h, then added 350ml of water, adjusted the pH to 5 with hydrochloric acid, quenched the reaction, then adjusted the pH to 10 with ammonia water, added 450ml of ethyl acetate for extraction, and the organic phase was extracted with water Wash and concentrate under reduced pressure to obtain the free oily state, which is 1-[5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrol-3-yl]-N-propylene glycol methylamine mono Free state of fumaric acid (compound V);
[0036] (2) Add 200ml of N,N-dimethylacetamide to compound V, stir to dissolve, add 14g of fum...
experiment example 1
[0044] Experimental Example 1 Water Solubility Evaluation
[0045] Sample: the compound shown in formula I and vonoprazan fumarate;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com