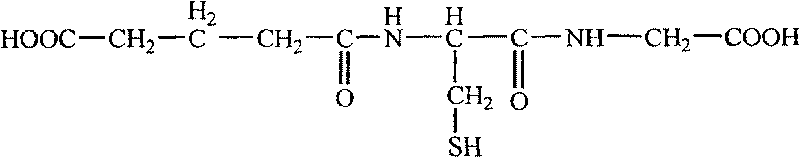
Medicine composition of glycyrrhizic acid or its salt and reduced glutathione
A technology of glutathione and composition, which is applied in the field of medicine to achieve the effects of ensuring safety, definite curative effect and reducing relative dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] The preparation of embodiment 1 Gan Gu composition powder injection
[0102] 1. Prescription:
[0103] Composition prescription of Gan Gu 1
[0104] Monoammonium Glycyrrhizinate 40g
[0105] Reduced Glutathione 300g
[0106] Glycine 400g
[0107] Cysteine Hydrochloride 20g
[0108] Mannitol 300g
[0109] Add sterile water for injection to 3000ml
[0110] A total of 1000 sticks were prepared
[0111] Gan Gu Composition Prescription 2
[0112] Diammonium Glycyrrhizinate 150g
[0113] Reduced Glutathione 300g
[0114] Mannitol 300g
[0115] Add sterile water for injection to 3000ml
[0116] A total of 1000 sticks were prepared
[0117] 2. Specific steps:
[0118] 1) First, aseptically treat the containers used for liquid preparation, antibiotic glass bottles, rubber stoppers, etc.
[0119] 2) Weigh the raw materials and auxiliary materials according to the prescription quantity.
[0120] 3) Take sterile water for injection with a dosing volume of 80%, ad...
Embodiment 2
[0128] Example 2 Preparation of Gan Gu Composition Aqueous Injection
[0129] 1. Prescription:
[0130] Gan Gu composition prescription 1
[0131] Monoammonium Glycyrrhizinate 40g
[0132] Reduced Glutathione 300g
[0133] Glycine 400g
[0134] Cysteine Hydrochloride 20g
[0135] Add water for injection to 10000ml
[0136] A total of 1000 sticks were prepared
[0137] Kangu Composition Prescription 2
[0138] Diammonium Glycyrrhizinate 150g
[0139] Reduced Glutathione 300g
[0140] Add water for injection to 10000ml
[0141] A total of 1000 sticks were prepared
[0142] 2. Specific steps:
[0143] 1) Dispose of the pipes and containers used for liquid preparation one day in advance, and rinse them with fresh water for injection before use.
[0144] 2) Take 80% of the water for injection, add the prescribed amount of monoammonium glycyrrhizinate (or diammonium glycyrrhizinate) and reduced glutathione, add amino acids according to the prescription, heat and stir...
Embodiment 3
[0153] Example 3 Preparation of Sodium Chloride Infusion of Sugar Grain Composition
[0154] 1. Prescription:
[0155] Composition prescription of Gan Gu 1
[0156] Monoammonium Glycyrrhizinate 40g
[0157] Reduced Glutathione 300g
[0158] Glycine 400g
[0159] Cysteine Hydrochloride 20g
[0161] Add water for injection to 100000ml
[0162] A total of 1000 bottles were prepared
[0163] Gan Gu Composition Prescription 2
[0164] Diammonium Glycyrrhizinate 150g
[0165] Reduced Glutathione 300g
[0167] Add water for injection to 100000ml
[0168] A total of 1000 bottles were prepared
[0169] 2. Specific steps:
[0170] 1) Dispose of the pipes and containers used for liquid preparation the day before, and rinse them with fresh water for injection before use.
[0171] 2) Add monoammonium glycyrrhizinate (or diammonium glycyrrhizinate) and reduced glutathione to water for injection with a dosing volum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com