Method for detecting chemical luminous analysis of chloro-phenol
A technology of chemiluminescence and chlorinated phenols, which is applied in the field of chemiluminescence reaction of determination materials, can solve the problems of low recovery rate, low determination sensitivity and cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Example 1 (determination of 2,4-dichlorophenol)
[0041] 1. Configure reagents
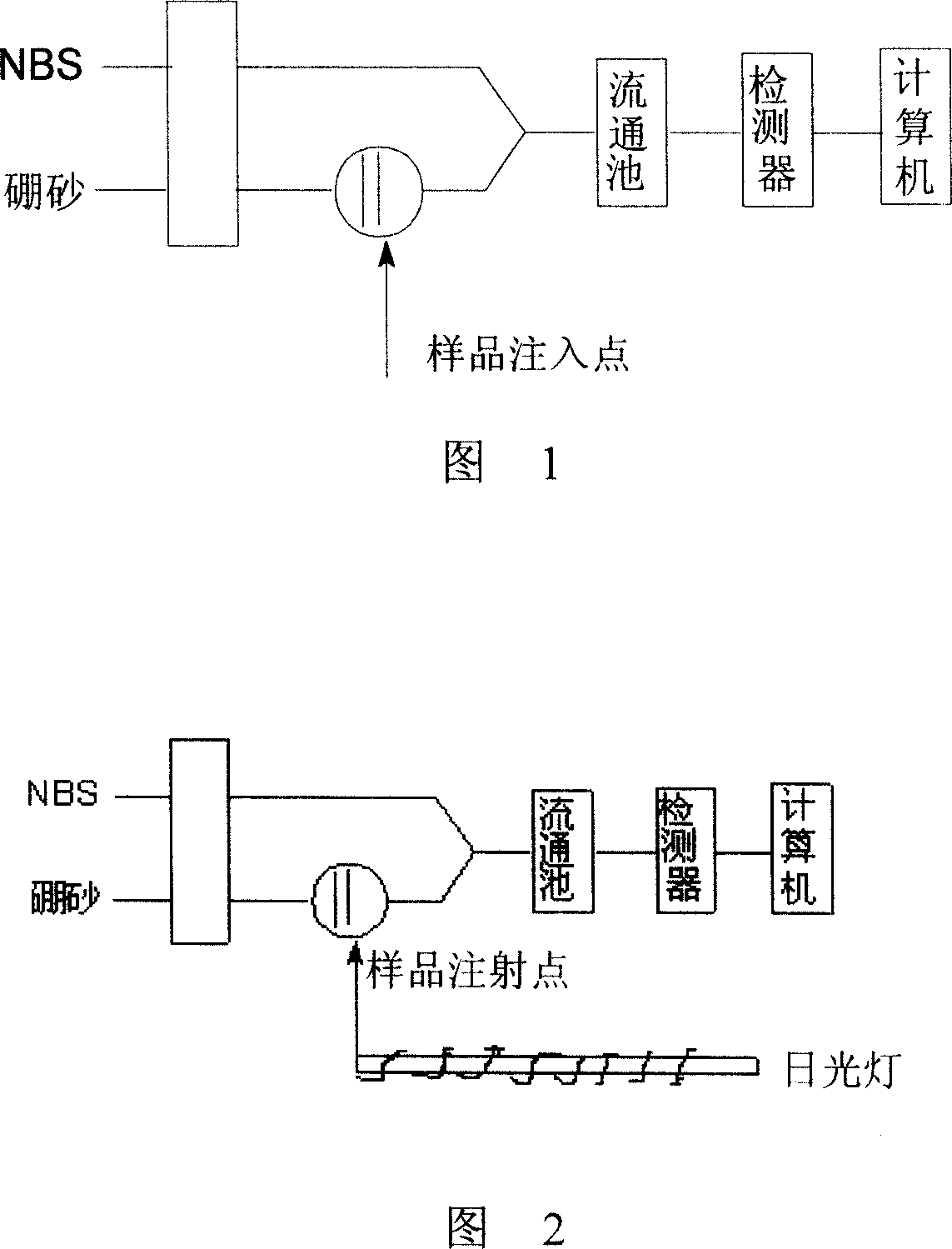
[0042] Sample stock solution: Accurately weigh 0.143g 2,4-dichlorophenol (analytical pure), use 1×10 -3 mol / L NaOH solution is dissolved and transferred to a 250mL brown volumetric flask, stored in a refrigerator (4°C) away from light, and diluted step by step when used; 5×10 -4 mol / L fluorescein stock solution: accurately weigh a certain amount of fluorescein, and use 1×10 -3 mol / LNaOH is dissolved and diluted step by step when used; 1.0×10 -3 mol / L N-bromosuccinimide (NBS) stock solution: 1.16×10 -1 mol / L Triton X-100 solution and 0.05mol / L borax solution, 0.25mol / L NaH 2 PO 4 are configured in the usual way. The reagents used were of analytical grade unless otherwise specified, and the water was high-purity deionized water.
[0043] 2. Experimental method
[0044] Take a certain amount of chlorophenol sample, add 1.0ml 5.0×10 -4 mol / L fluorescein, 5.0ml 0.25mol / LNaH 2 PO 4 Buf...
example 2
[0054] Example 2 (2, the determination of 4-chlorophenol)
[0055] 1. Configure reagents
[0056] Sample stock solution: Accurately weigh 0.143g 2,4-dichlorophenol (analytical pure), use 1×10 -3 mol / L NaOH solution is dissolved and transferred to a 250mL brown volumetric flask, stored in a refrigerator (4°C) away from light, and diluted step by step when used; 5×10 -4 mol / L methylene blue stock solution: accurately weigh a certain amount of methylene blue, and use 1×10 -3 mol / L NaOH is dissolved and diluted step by step when used; 1.0×10 -3 mol / L N-bromosuccinimide (NBS) stock solution, 1.16×10 -1 mol / L Triton X-100 solution and 0.05mol / L borax solution, 0.25mol / L NaH 2 PO 4 are configured in the usual way. The reagents used were of analytical grade unless otherwise specified, and the water was high-purity deionized water.
[0057] 2. Experimental method
[0058] Take a certain amount of chlorophenol sample, add 2.5ml5.0×10 -4 mol / L methylene blue, 5.0ml0.25mol / L NaH ...
example 3
[0068] Example 3 (2,4, the determination of 6-trichlorophenol)
[0069] 1. Configure reagents
[0070] Sample stock solution: Accurately weigh 0.173g 2,4,6-trichlorophenol (analytical pure), use 1×10 -3 mol / L NaOH solution is dissolved and transferred to a 250mL brown volumetric flask, stored in a refrigerator (4°C) away from light, and diluted step by step when used; 5×10 -4 mol / L fluorescein stock solution: accurately weigh a certain amount of fluorescein, and use 1×10 -3 mol / L NaOH is dissolved and diluted step by step when used; 1.0×10 -3 mol / L dibromohydantoin stock solution, 1.16×10 -1mol / L Triton X-100 solution and 0.05mol / L borax solution, 0.25mol / L NaH 2 PO 4 are configured in the usual way. The reagents used were of analytical grade unless otherwise specified, and the water was high-purity deionized water.
[0071] 2. Experimental method
[0072] Take a certain amount of chlorophenol sample, add 1.0ml 5.0×10 -4 mol / L fluorescein, 5.0ml0.25mol / L NaH 2 PO 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com