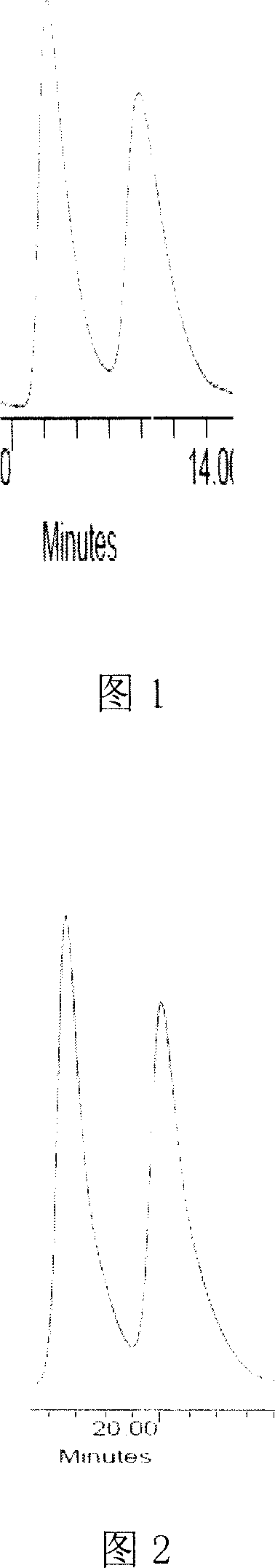
Teicoplanin p-chlorophenyl isocyanate chiral stationary phase filling and method for preparing same
A technology of chlorophenylisocyanate and chiral stationary phase, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Silanization of carrier silica gel
[0035] Weigh 10 grams of Kromasil spherical silica gel carrier (particle size 5 μm, pore size 10 nm) with 3 mol L -1 After hydrochloric acid was refluxed for 4 hours, it was washed with water to neutrality, dried in vacuum, and 10ml of 3-aminopropyltriethoxysilane was added with anhydrous toluene as a solvent, then refluxed for 8 hours, cooled to room temperature, filtered, washed with toluene and methanol, Vacuum drying to obtain 3-aminopropyl silica gel;
[0036] 2) Synthesis of bonded chiral stationary phase
[0037] Take 3 grams of the above dried 3-aminopropyl silica gel, add it to anhydrous toluene, add 2ml of 1,6-diisocyanate n-hexane dropwise under ice bath and nitrogen protection, heat the mixture to 65°C for 2 hours, and cool to After room temperature, remove the solvent and 1,6-diisocyanate n-hexane, add 100ml of anhydrous pyridine solution containing 1g of teicoplanin, stir and react at 65°C for 10 hours under nitrog...
Embodiment 2
[0039] 1) Silanization of carrier silica gel
[0040]Weigh 10 grams of Lichrosorb amorphous silica gel carrier (particle size 5 μm, pore size 10 nm) with 5 mol L -1 Hydrochloric acid was refluxed for 6 hours, washed with water until neutral, dried in vacuum, using anhydrous toluene as solvent, added 3-aminopropyltriethoxysilane, refluxed for 10 hours, cooled to room temperature, filtered, washed with toluene and methanol, and dried in vacuum Get 3-aminopropyl silica gel;
[0041] 2) Synthesis of bonded chiral stationary phase
[0042] Take 3 grams of the above-mentioned dry 3-aminopropyl silica gel, add it to anhydrous toluene, add 3ml of 1,6-diisocyanate n-hexane dropwise under ice bath and nitrogen protection, heat the mixture to 70°C for 3 hours, and cool to Remove the solvent and 1,6-diisocyanate n-hexane after room temperature, add 100ml of anhydrous pyridine solution containing 2g of teicoplanin, stir and react at 70°C for 10 hours under nitrogen protection, filter, wa...
Embodiment 3
[0044] 1) Silanization of carrier silica gel
[0045] Weigh 10 grams of Lichrospher spherical silica gel carrier (particle size 5 μm, pore size 10 nm) with 5 mol L -1 Hydrochloric acid was refluxed for 4 hours, washed with water until neutral, dried in vacuo, using anhydrous toluene as solvent, added 3-aminopropyltriethoxysilane, refluxed for 8 hours, cooled to room temperature, filtered, washed with toluene and methanol, and dried in vacuo Get 3-aminopropyl silica gel;
[0046] 2) Synthesis of bonded chiral stationary phase
[0047] Take 3 grams of the above dried 3-aminopropyl silica gel, add it to anhydrous toluene, add 4ml of 1,6-diisocyanate n-hexane dropwise under ice bath and nitrogen protection, heat the mixture to 75°C for 3 hours, cool to After room temperature, remove the solvent and 1,6-diisocyanate n-hexane, add 100ml of anhydrous pyridine solution containing 2g of teicoplanin, stir and react at 75°C for 12 hours under nitrogen protection, filter, wash with pyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com