Method of making liposomes, liposome compositions made by the methods, and methods of using the same
a technology of liposome composition and composition, applied in the field of making liposome composition, can solve the problems of other limitations of the related art, and achieve the effects of preventing oxidative stress or damage, and high dose of liposome composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Sodium Ascorbate Entrapped Liposomes
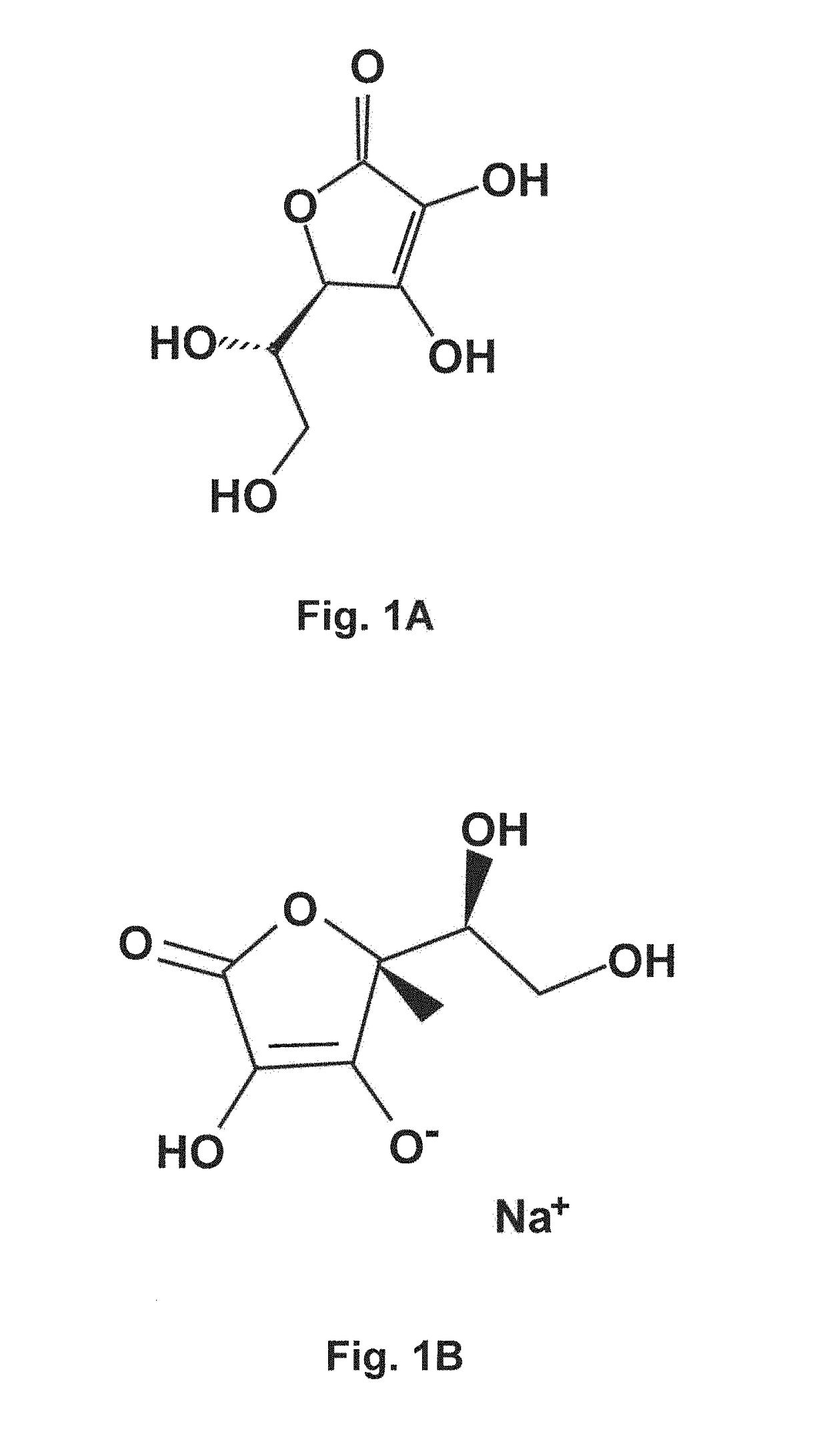
[0141]97 g Phospholipon 50IP (a non-GMO vesicle-forming phosphatidylcholine lipid available from Lipoid) was solubilized in 56.3 g 200 proof alcohol at room temperature. Care was taken to ensure that all lipids were completely solubilized and no undissolved lipid remained. 109,5 g sodium ascorbate and 26.4 mg EDTA was separately solubilized in 251 g reverse osmosis-treated water at room temperature with normal agitation. The pH of the Na ascorbate-EDTA solution was adjusted to 6.3 The sodium ascorbate-EDTA solution was filtered with a 0.2 μm filter to remove dust particles and other contaminants. Streams of the lipid solution were slowly injected into the vitamin solution with continuous vortexing with a mixer. After all the lipid solution was added, lipid hydration was allowed to continue about 15 minutes with frequent mixing. At this point, the liposomes had formed in a smooth, translucent fluid.
[0142]2.2 g xanthan gum (available ...
example 2
Comparison of Empirical Liposomes and LivOn Liposomes
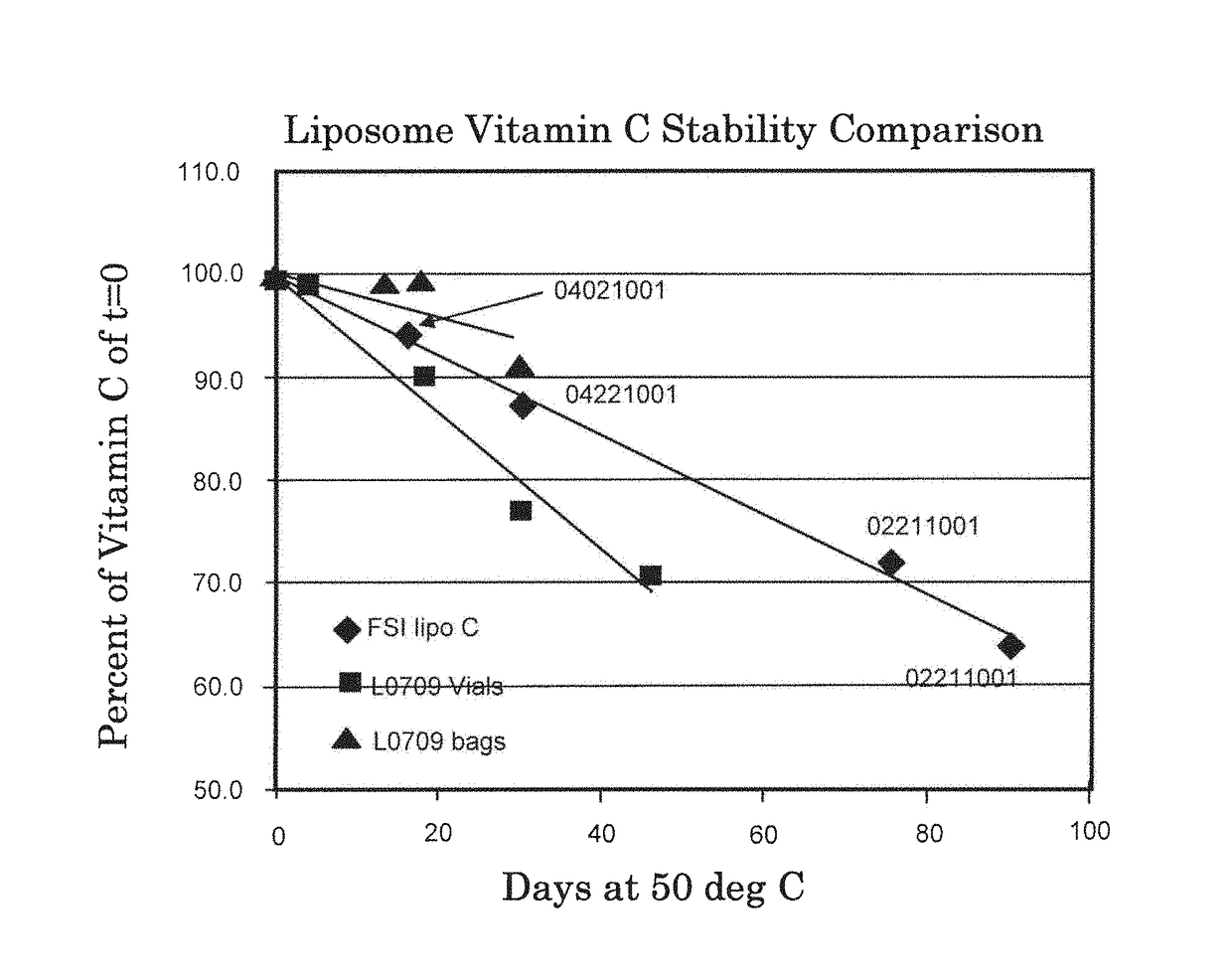
[0143]Several batches of Lypo-Spheric™ Vitamin C liposomes were obtained from LivOn Laboratories. According to the product ingredient table, each 5.7 mL packet of Lypo-Spheric™ Vitamin C liposomes contains 1,000 mg of sodium ascorbate and 1.0 g of essential phospholipids. The Lypo-Spheric™ Vitamin C liposomal product ranged in color from light orange to brownish. A second commercial product, Liposomal Vitamin C liposomes, were obtained from Empirical Laboratories. According to the product ingredients table, each 4 mL serving size of Empirical Liposomal Vitamin C liposomes contains 1000 mg sodium ascorbate and 400 mg of phosphatidylcholine.
[0144]The LivOn and Empirical liposome formulations were compared for sodium ascorbate amounts, particle size, amount of dry material and amount of lipid (estimated) with the results shown in Table 2. The Empirical liposomes appeared dilute in comparison to the LivOn liposomes. The Empirical lipo...
example 3
Comparison of Cold Process Liposomes to LivOn Laboratories Liposomes
[0152]Cold process liposomes were prepared essentially as described in Example 1, Lypo-Spheric™ Vitamin C liposomes were obtained from LivOn Laboratories. Various properties of the liposome formulations were determined with the results shown in Table 3.
[0153]The weight percent of ascorbic acid in the liposome formulations was determined by HPLC. The alcohol content was determined by gas chromatography. Mean particle size was determined by dynamic light scattering. Sodium ascorbate encapsulation was determined by filtration and HPLC as described in Example 2.
[0154]
TABLE 3Comparison of Cold Process Liposomes and LivOn LiposomesCold ProcessLivOn LaboratoriesLiposomesLypo-Spheric ™ Vitamin CVitamin C (ascorbic19.6%16.5%acid) %Phospholipids (wt %)18.8%18.7%Alcohol 10-11% 9-10%Mean particle size300-450 800-1200(nm)Particle size150-200600-900distribution SD (nm)Sodium ascorbate3535encapsulation (%)pH6.36.0-6.5Vitamin C wt ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean particle size diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com