Oxo-M and 4-PPBP induction of tenogenic differentiation of perivascular tendon stem cells
a perivascular tendon and stem cell technology, applied in the field of oxom and 4ppbp induction of tenogenic differentiation of perivascular tendon stem cells, can solve the problems of limited clinical use of ctgf as a tendon injury therapy, difficult clinical adoption and regulatory approval, and crucial barriers to cell transplantation, etc., to achieve more aligned collagen structure, improve healing, and reduce gaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
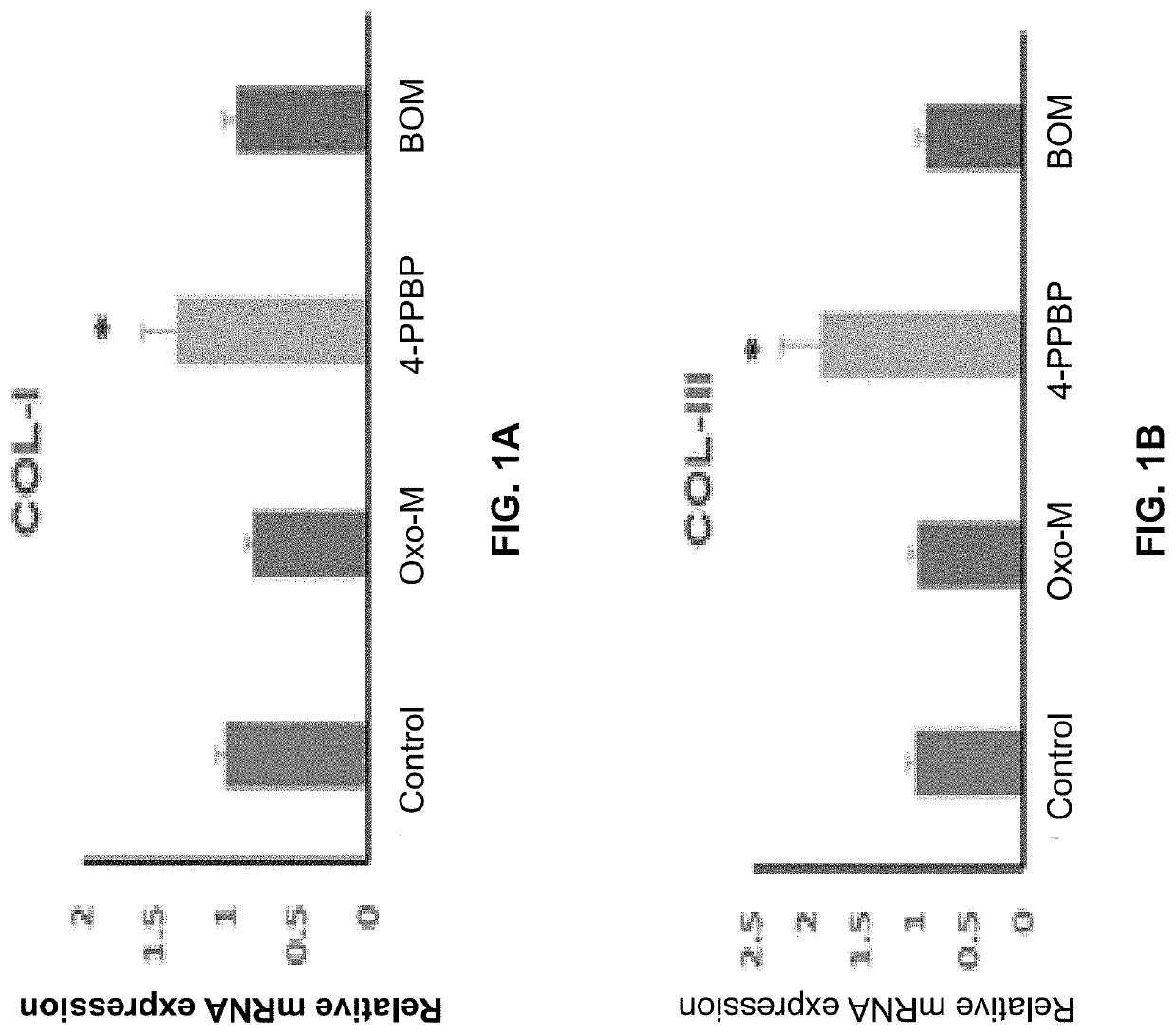
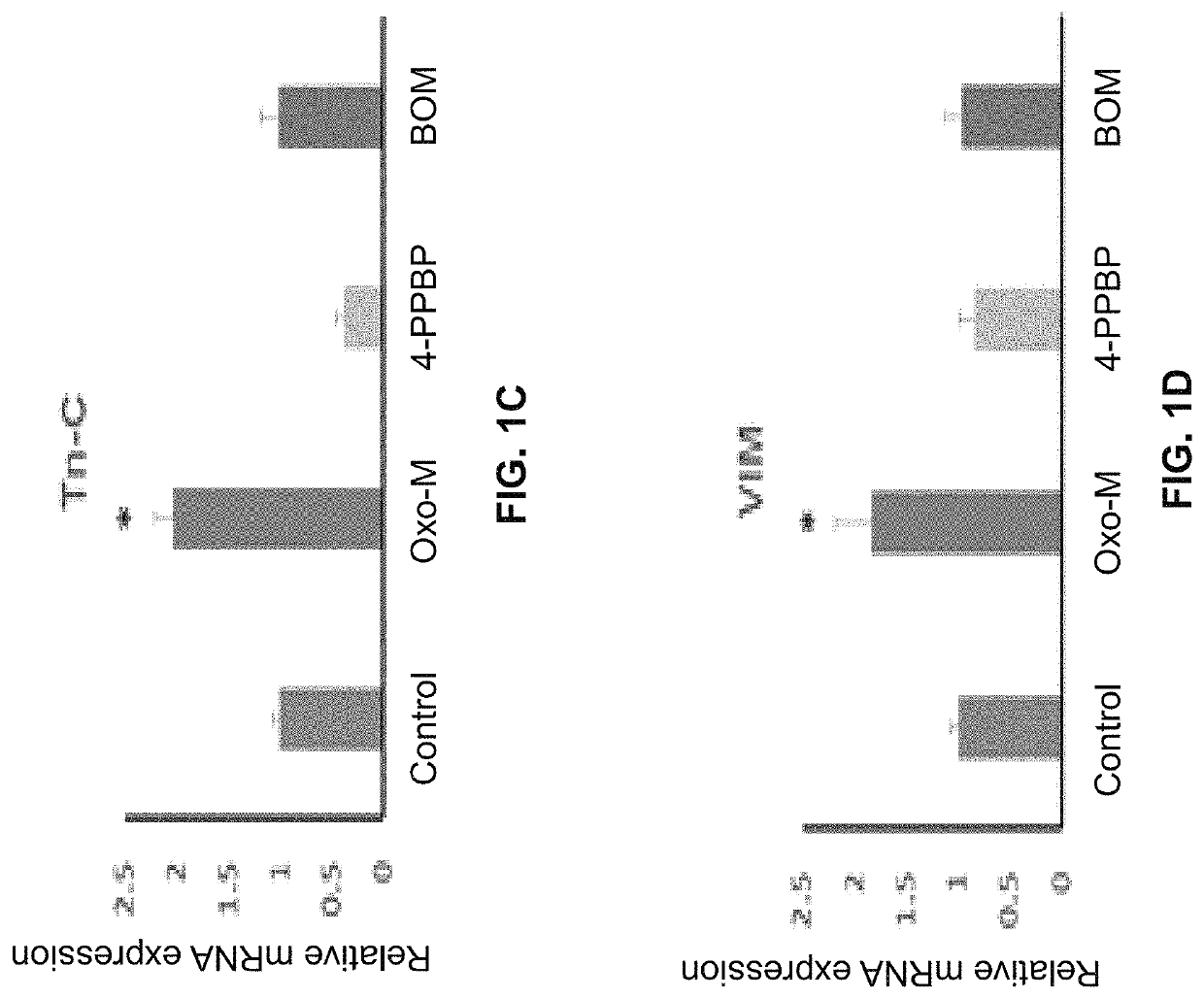
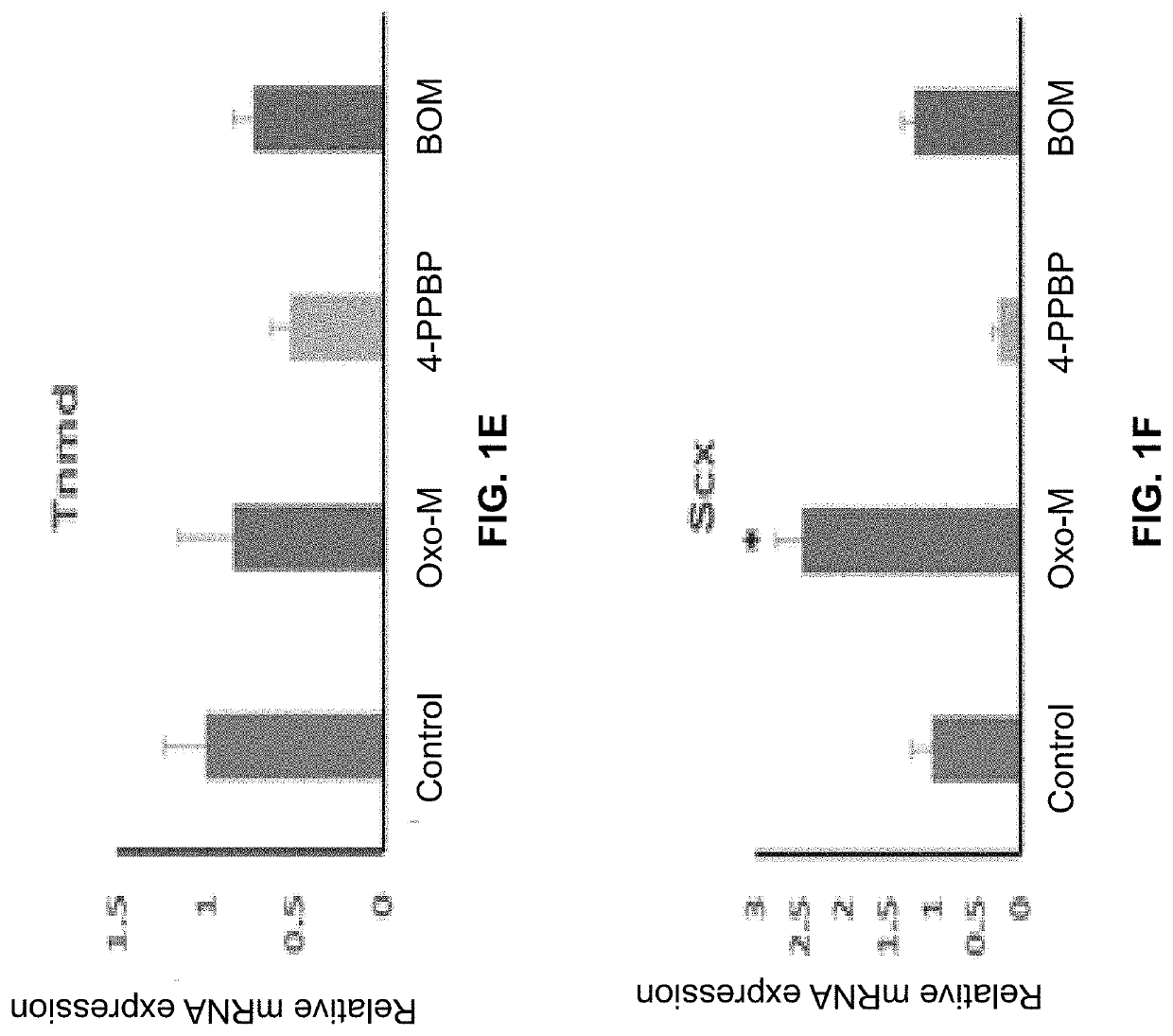
[0141]The following example shows a combination of Oxo-M and 4-PPBP induces tenogenic differentiation of perivascular tendon stem cells.
[0142]Perivascular tendon stem cells (PTSCs) were isolated from patellar tendons of 12 week-old Sprague-Dawley rats by sorting cells with strong surface expression of CD146, following established protocol (Lee, et al., 2015, supra). Culture-expanded PTSC (P2-3) were then treated with selected FAK and ERK1 / 2 agonists, including Oxotremorine M (Oxo-M) (1 mM), PPBP maleate (4-PPBP) (10 μM), Phenylarsine oxide (PAO) (2 μM), and [Tyr4]-Bombesin (BOM) (10 nM), and SKF-83959 (20 μM). A single concentration was tested for each molecule as a first screening study. After 1 week, tendon-related mRNA expressions, including collagen I and III, tenascin-C (Tn-C), vimentin (VIM), tenomodulin (Tnmd), and scleraxis (Scx), were measured.
[0143]Results showed that Oxo-M provided significant increases in Tn-C, VIM, and Scx, while 4-PPBP elevated expressions of COL-I and...
example 2
[0147]This Example shows that delivery in vivo of a combination of Oxo-M and 4-PPBP in a rat tendon defect model showed improved tendon healing. The animal model was used to study the in vivo response and development of these tissues from endogenous cells stimulated by Oxo-M and 4-PPBP delivery.
[0148]This study was designed to investigate effect of Oxo-M and 4-PPBP on tendon healing. Animals were given Oxo-M alone (1 mM), 4-PPBP alone (10 μM), and a combination of Oxo-M (1 mM)+4-PPBP (10 μM) along with fibrin gel as a vehicle after patellar tendon transection. Fibrin alone served as a vehicle control. Multiple time points (1 and 2 weeks) were selected to follow up the process of healing and remodeling of tendon. Tissue healing, matrix formation and remodeling, and functional restoration were examined using histology, immunohistochemistry, and mechanical testing (tensile) as per prior methods (Lee, et al., J. Clin. Invest. 120:3340-3349, 2010; Lee, et al., Lancet 376:440-448, 2010; L...
example 3
[0161]This Example shows that delivery in vivo of a combination of Oxo-M and 4-PPBP in a rat supraspinatus tendon injury model showed improved tendon healing. The animal model was used to study the in vivo response and development of these tissues from endogenous cells stimulated by Oxo-M and 4-PPBP delivery.
[0162]This study was designed to investigate effect of a combination of Oxo-M (1 mM)+4-PPBP (10 μM) along with fibrin gel as a vehicle after supraspinatus tendon transection. Fibrin alone served as a vehicle control. Four weeks post-op was selected to follow up the process of healing and remodeling of tendon. Tissue healing, matrix formation and remodeling, and functional restoration were examined using histology as per prior methods (Lee, et al., J. Clin. Invest. 120:3340-3349, 2010; Lee, et al., Lancet 376:440-448, 2010; Lee, et al., Sci. Transl. Med. 6(266):266ra171, 2014; Lee et al., 2015, supra).
[0163]The study was designed as follows:
[0164]1. Rat supraspinatus tendon was t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com