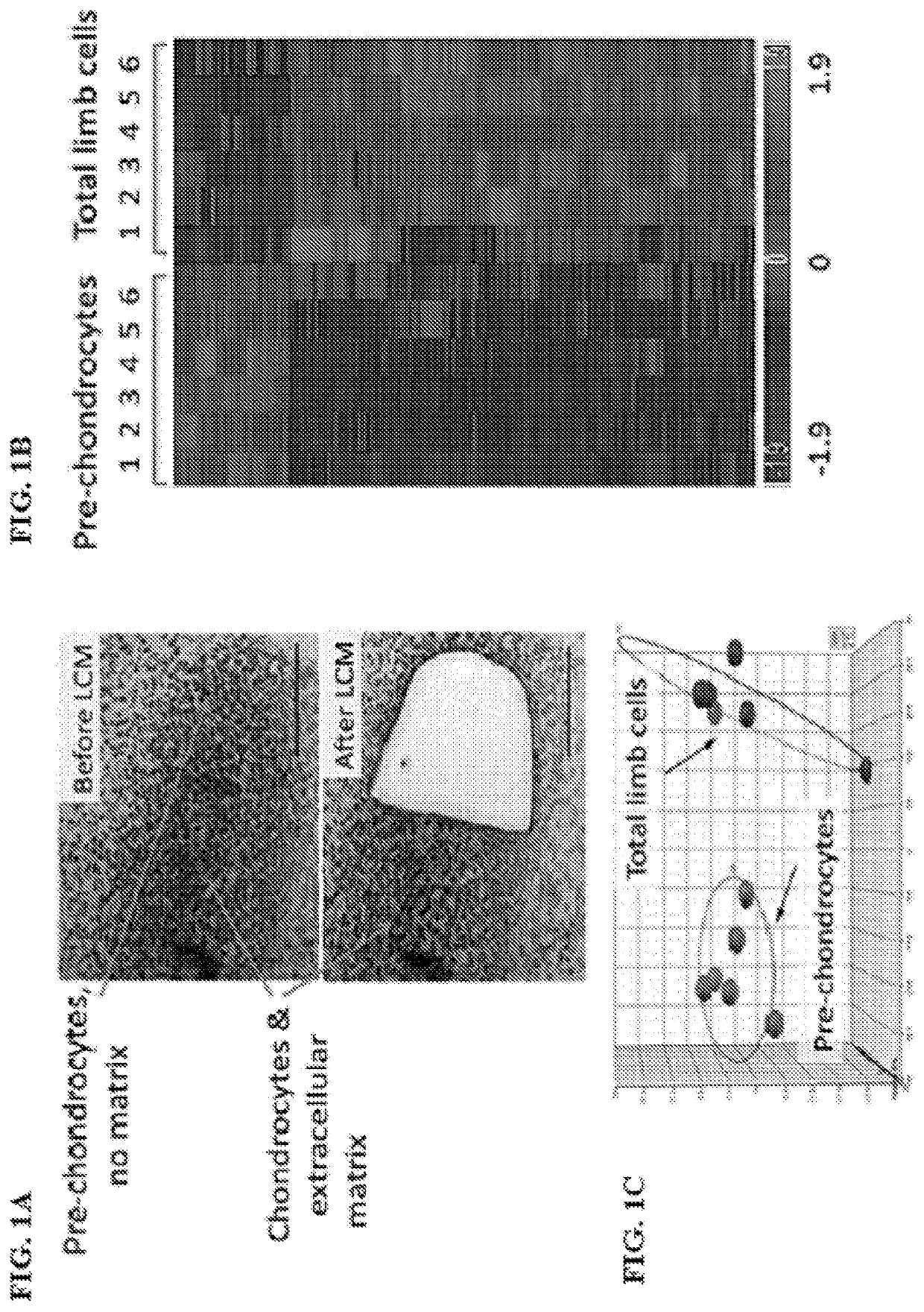
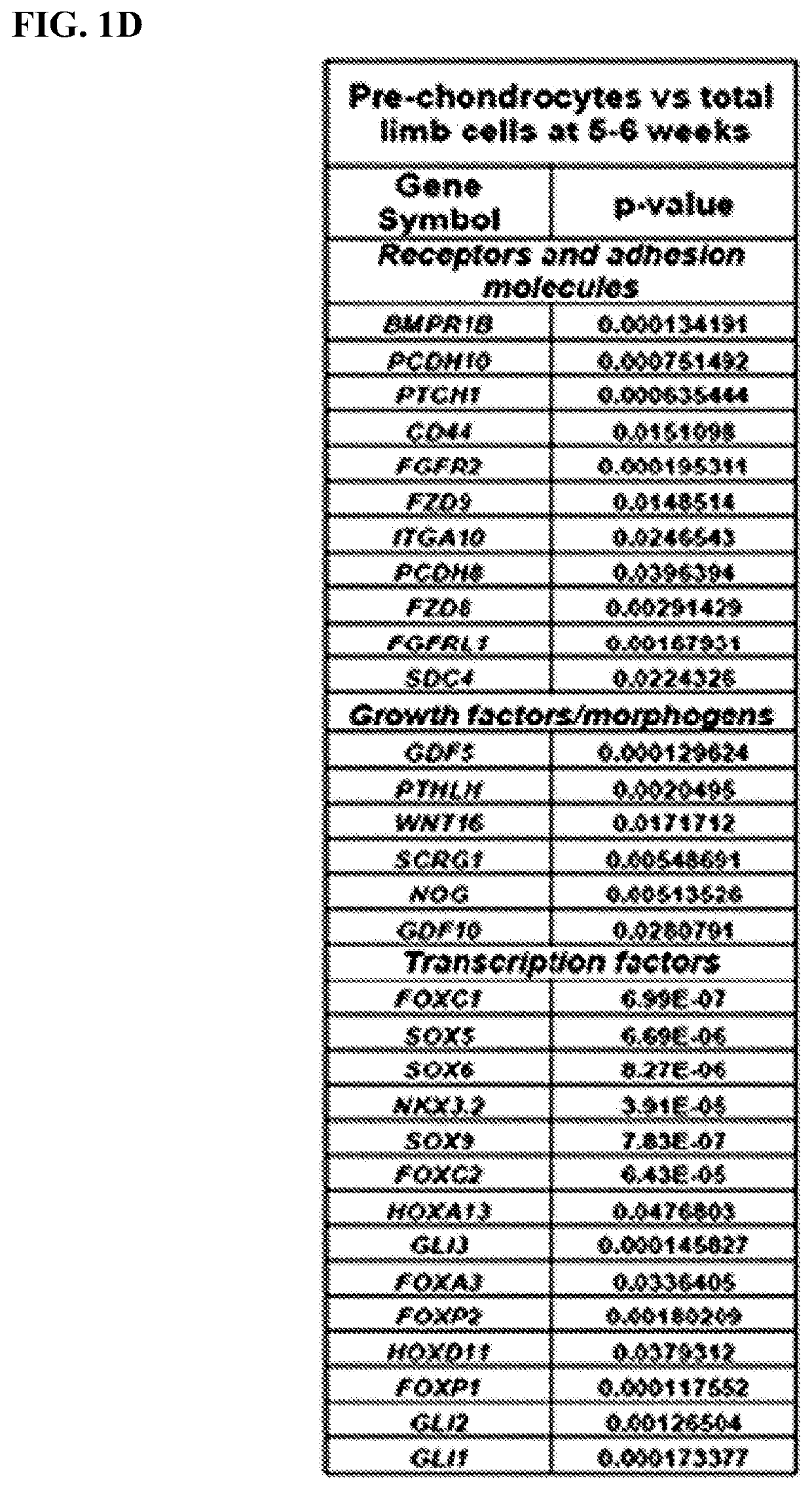
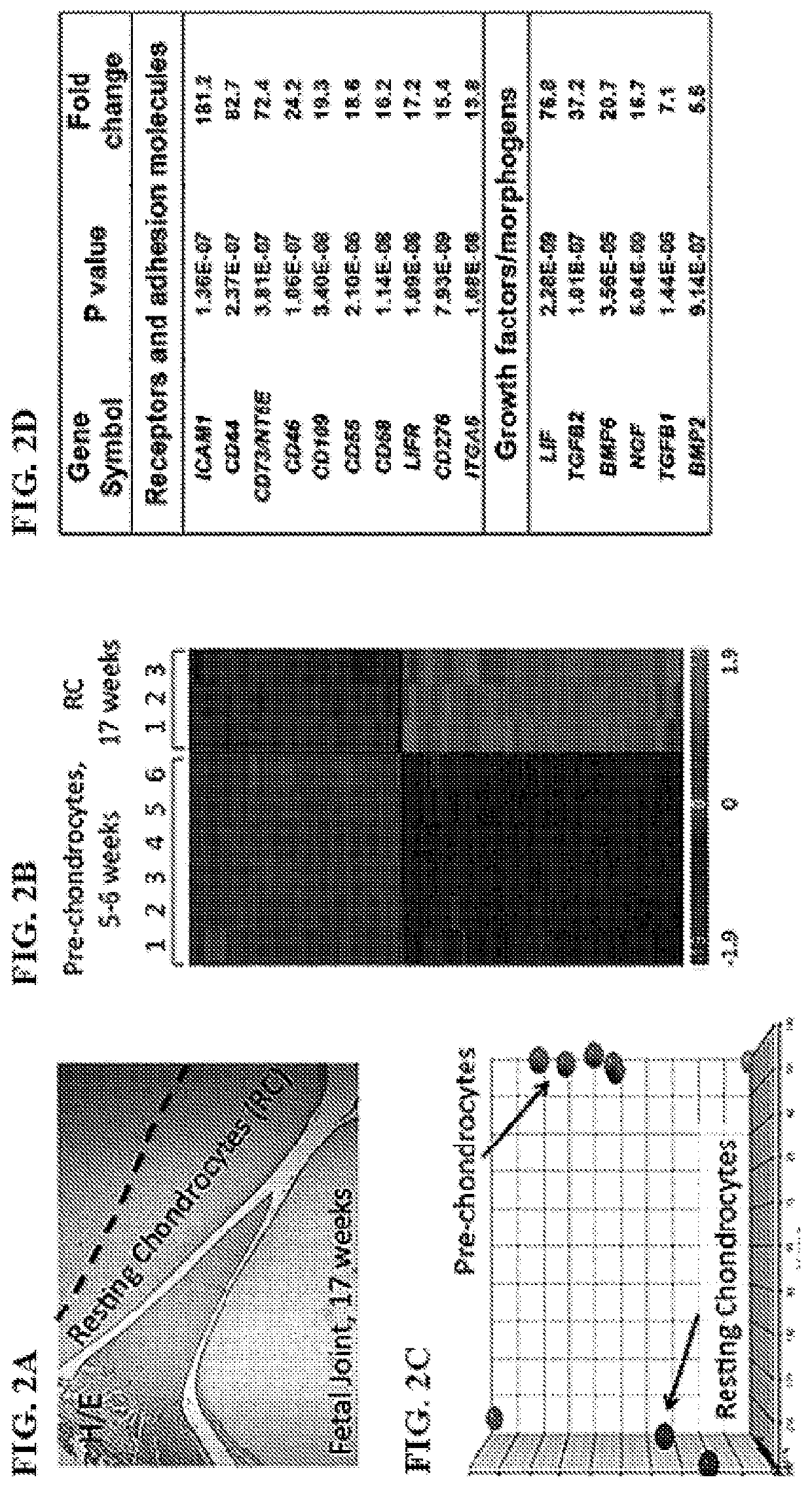
Small molecules that enable cartilage rejuvenation
a small molecule and cartilage technology, applied in the direction of organic chemistry, organic active ingredients, drug compositions, etc., can solve the problems of limited cartilage regeneration in humans, osteoarthritis, and inability to regenerate cartilage, and achieve the effect of increasing the secretion of cartilaginous matrix in cartilag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
embodiment p1
[0334]A method for activating a proliferative program in competent adult chondrocytes, said method comprising contacting a competent adult chondrocyte with an activating compound, said activating compound capable of increasing expression of p-STAT3 and c-Myc in said competent adult chondrocyte.
embodiment p2
[0335]The method according to embodiment P1, wherein said activating compound has the structure of Formulae (PI):
[0336]
wherein R1 is substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; R2 is substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; and R3 is substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl; or Formula (PII):
[0337]
wherein R4 is substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl,...
embodiment p3
[0338]The method according to embodiment P2, wherein said activating compound has the structure of Formula (PIa):
[0339]
wherein n is an integer from 0 to 5; and R6 at each occurrence is independently halogen, —CF3, —CN, —OH, —NH2, —COOH, —CONH2, —NO2, —SH, —SO2Cl, —SO3H, —SO4H, —SO2NH2, —NHNH2, —ONH2, —NHC═(O)NHNH2, —NHC═(O) NH2, —NHSO2H, —NHC═(O)H, —NHC(O)—OH, —NHOH, —OCF3, —OCHF2, substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com