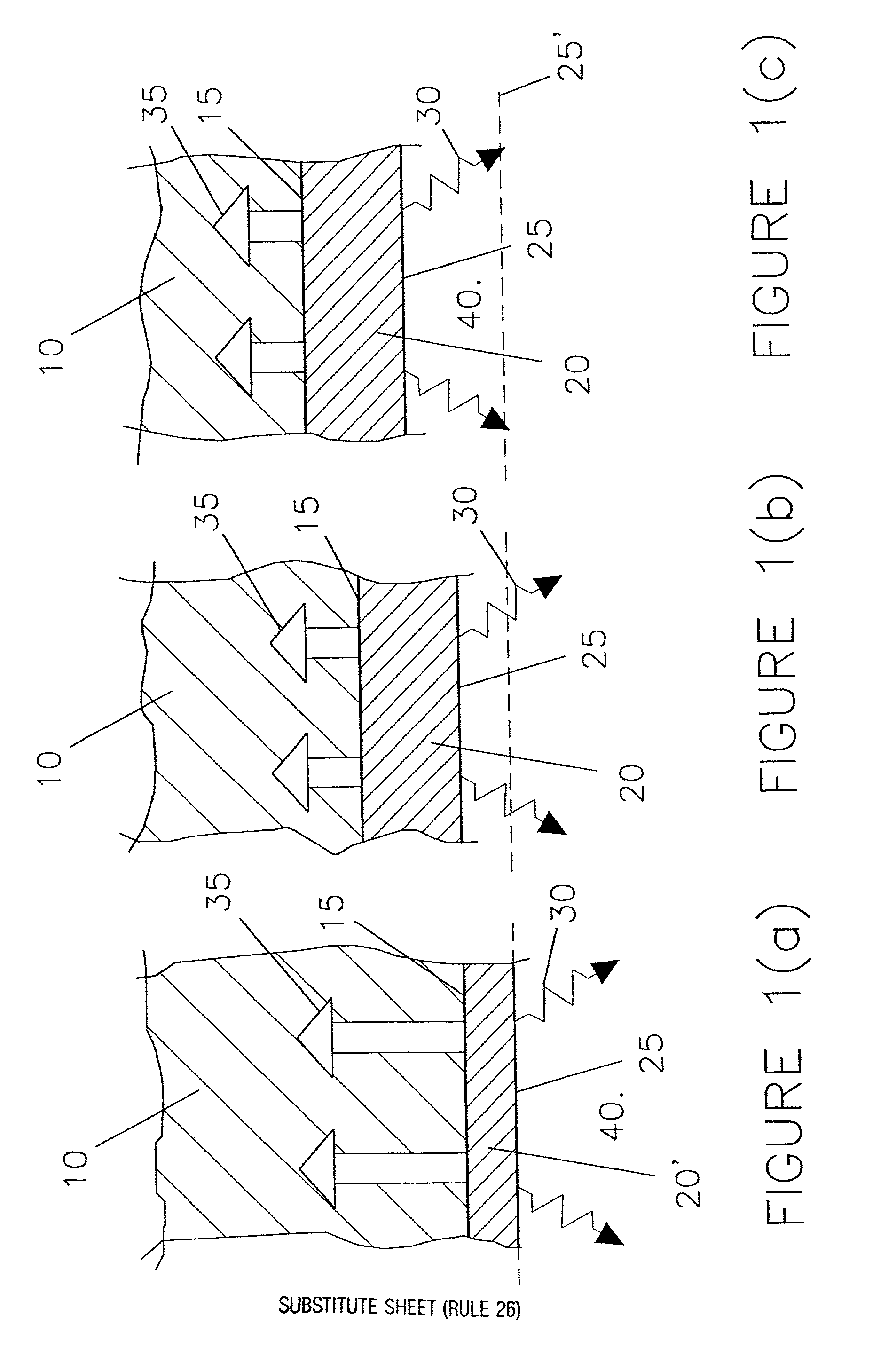
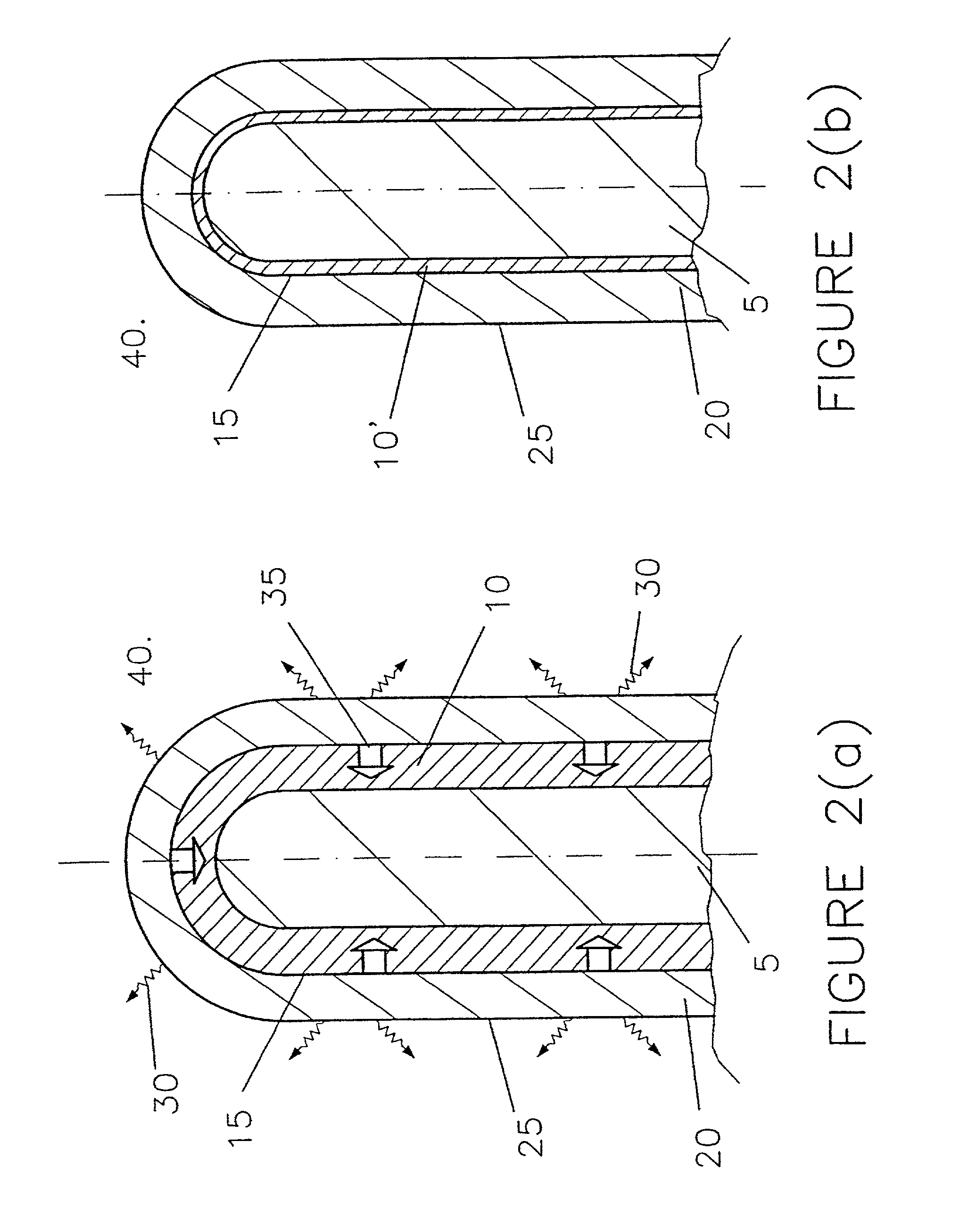
Slow consumable non-carbon metal-based anodes for aluminium production cells
a production cell, non-carbon technology, applied in the direction of electrochemical machining apparatus, electrolysis components, electrolysis coatings, etc., can solve the problem of difficult to achieve full protection of alloy substrates, and achieve the effect of reducing the frequency of anode replacement and reducing carbon-generated pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0071] A non-carbon metal-based anode according to the invention was obtained from a 15.times.15.times.80 mm sample of a nickel-iron based alloy. The sample was made of cast alloy consisting of 79 weight % nickel, 10 weight % iron and 11 weight % copper.
[0072] The sample was pre-oxidised in air at about 1100.degree. C. for 5 hours in a furnace to form the anode with a pre-oxidised surface layer.
[0073] After pre-oxidation, the anode was immersed in molten cryolite contained in a laboratory scale cell. The molten cryolite contained approximately 6 weight % of dissolved alumina. Current was passed through the anode sample at a current density of 0.5 A / cm.sup.2. After 100 hours, the anode was extracted from the cell for analysis.
[0074] The anode was crack-free and its dimensions remained substantially unchanged. On the surface of the anode a well adherent oxide surface layer of a thickness of about 0.6 mm had grown providing an adequate protection.
example 3
[0075] This Example illustrates the wear rate of the nickel-iron containing anode of Example 2 and is based upon observations made on dissolution of nickel-based samples in a fluoride-based electrolyte.
[0076] An estimation of the wear rate is based on the following parameters and assumptions:
[0077] With a current density of 0.7 A / cm.sup.2 and a current efficiency of 94% an aluminium electrowinning cell produces daily 53.7 kg aluminium per square meter of active cathode surface.
[0078] Assuming a contamination of the produced aluminium by 200 ppm of nickel, which corresponds to the experimentally measured quantities in typical tests, the wear rate of a nickel-iron sample corresponds to approximately 1.2 micron / day. Therefore, it will theoretically take about 80 to 85 days to wear 0.1 mm of the anode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com