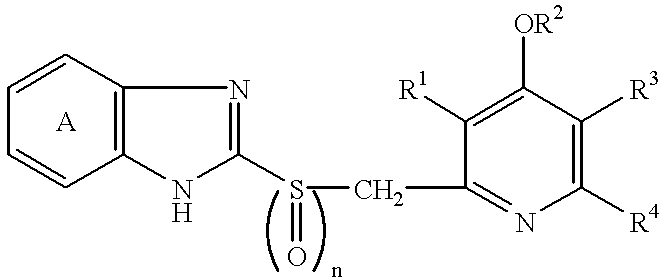
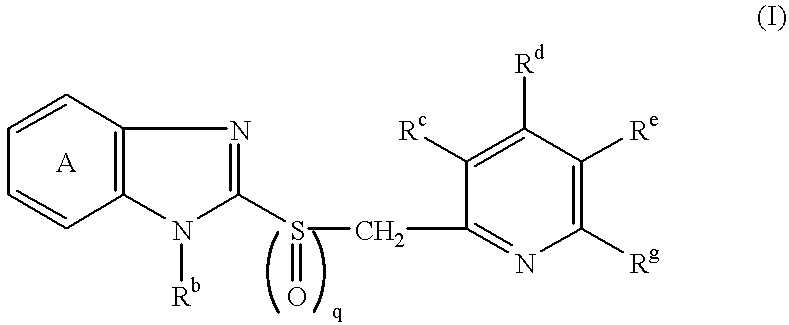
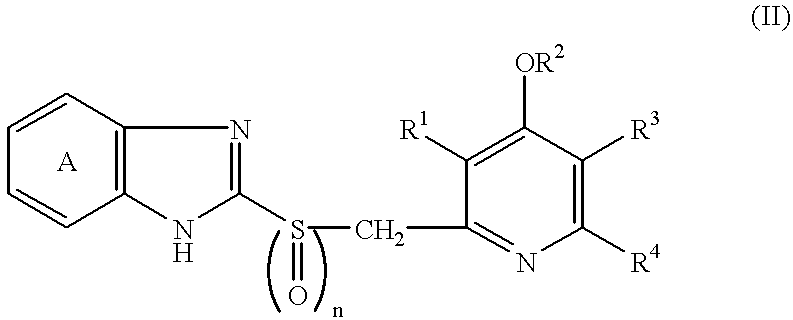
Formulation comprising antibacterial substance and antiulcer substance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 2
[0146] Production of Gastrointestinal Mucosa-Adherent Solid Preparation Containing Compound A
[0147] 12 g of behenic acid hexa(tetra)glyceride (HB-310, trade name, produced by Sakamoto Yakuhin K.K.) was thermally molten at 85.degree. C., and 4 g of Compound A and 4 g of a poly (acrylic acid) (Hiviswako 104, Wako Pure Chemical Industries) were added, followed by stirring at 80.degree. C. for 15 minutes, to yield a dispersion. The resulting molten mixture was added drop by drop to an aluminum disk of 15 cm in diameter rotating at 1,500 rpm at 10 g / min to yield spherical fine subtilaes which pass through a 30-mesh sieve but not through an 80-mesh sieve (hereinafter referred to as 30 / 80 mesh).
reference example 3
[0148] Production of Gastrointestinal Mucosa-Adherent Solid Preparation Containing Amoxicillin (AMOX)
[0149] 75 g of behenic acid hexa(tetra)glyceride (HB-310, trade name, produced by Sakamoto Yakuhin K.K.) was thermally molten at 74.degree. C., and 10 g of AMOX and 15 g of a poly (acrylic acid) (Hiviswako 104, Wako Pure Chemical Industries) were added, followed by stirring at 74.degree. C. for 15 minutes, to yield a dispersion. The resulting molten mixture was added drop by drop to an aluminum disk of 15 cm in diameter rotating at 2,400 rpm at 10 g / min to yield 30 / 80 mesh spherical fine subtilaes.
[0150] For oral administration in humans or non-human animals, 100 mg of the above fine subtilaes was packed in No. 4 capsules to yield a capsular preparation.
reference example 4
[0151] Production of Gastrointestinal Mucosa-Adherent Solid Preparation Containing Compound A
[0152] 27.5 g of behenic acid hexa(tetra)glyceride (HB-310, trade name, produced by Sakamoto Yakuhin K.K.) was thermally molten at 85.degree. C., and 8 g of Compound A, 7.5 g of a poly (acrylic acid) (Hiviswako 104, Wako Pure Chemical Industries) and 10 g of tartaric acid were added, followed by stirring at 80.degree. C. for 15 minutes, to yield a dispersion. The resulting molten mixture was added drop by drop to an aluminum disk of 15 cm in diameter rotating at 2,400 rpm at 10 g / min to yield 30 / 80 mesh spherical fine subtilaes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com