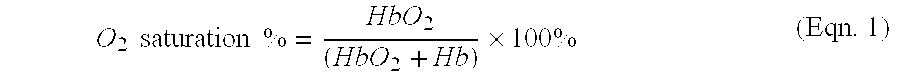Method for non-invasive spectrophotometric blood oxygenation monitoring
a spectrophotometric and blood oxygenation monitoring technology, applied in the field of non-invasive spectrophotometric blood oxygenation monitoring, can solve the problems of inability to provide pulse oximetry any information about venous blood, inability to provide absolute measurement of chromophore concentration (c), and inability to provide a means for determining the total level of blood oxygen saturation within biological tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
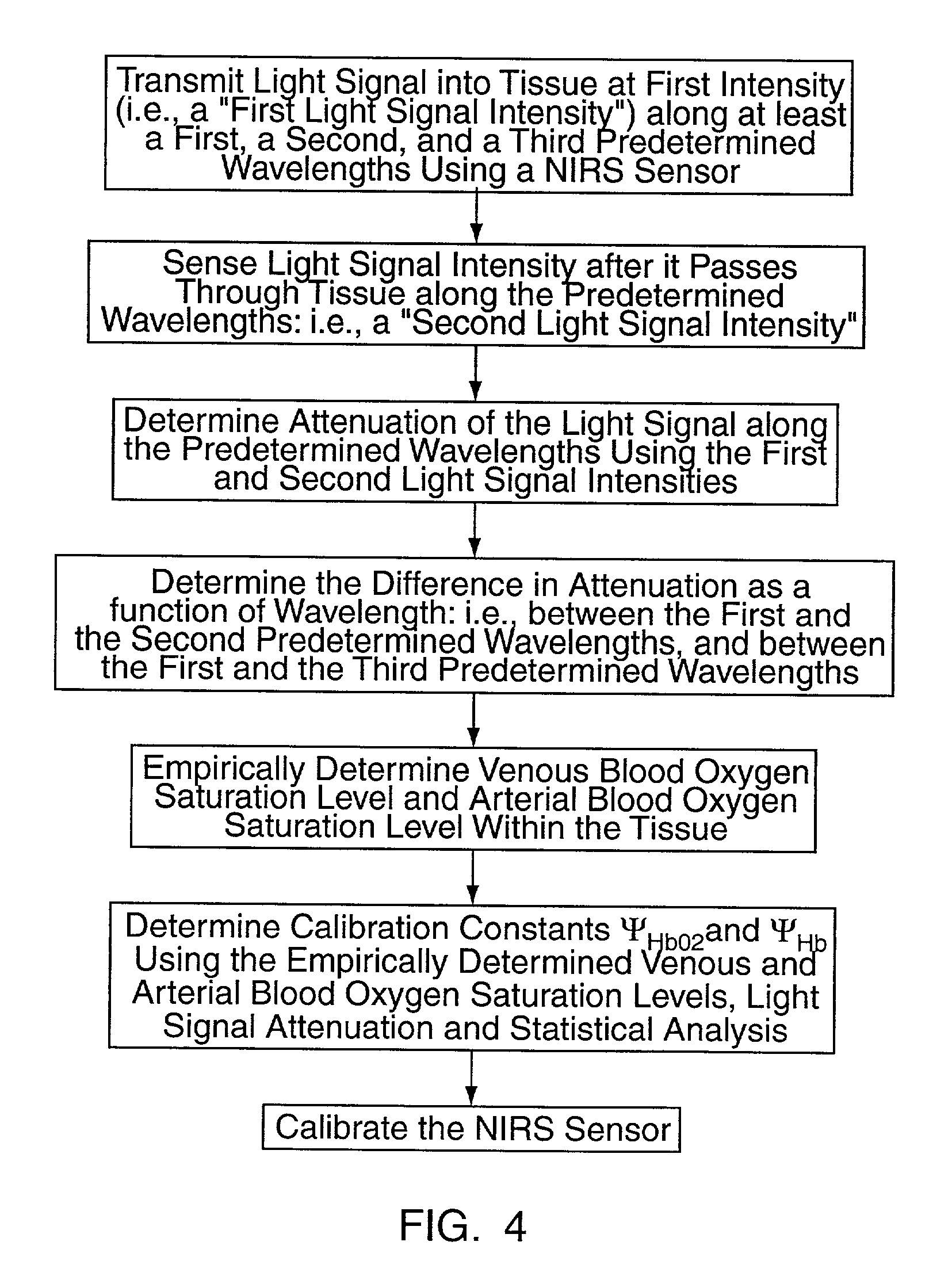
[0036] The present method of and apparatus for non-invasively determining the blood oxygen saturation level within a subject's tissue is provided that utilizes a near infrared spectrophotometric (NIRS) sensor that includes a transducer capable of transmitting a light signal into the tissue of a subject and sensing the light signal once it has passed through the tissue via transmittance or reflectance. The present method and apparatus can be used with a variety of NIRS sensors. The NIRS sensor described below, which is the subject of co-pending U.S. patent application Ser. No. 09 / 434,146 filed Nov. 4, 1999 commonly assigned with the present application, discloses a preferred NIRS sensor. The present method is not limited to use with this preferred NIRS sensor, however.
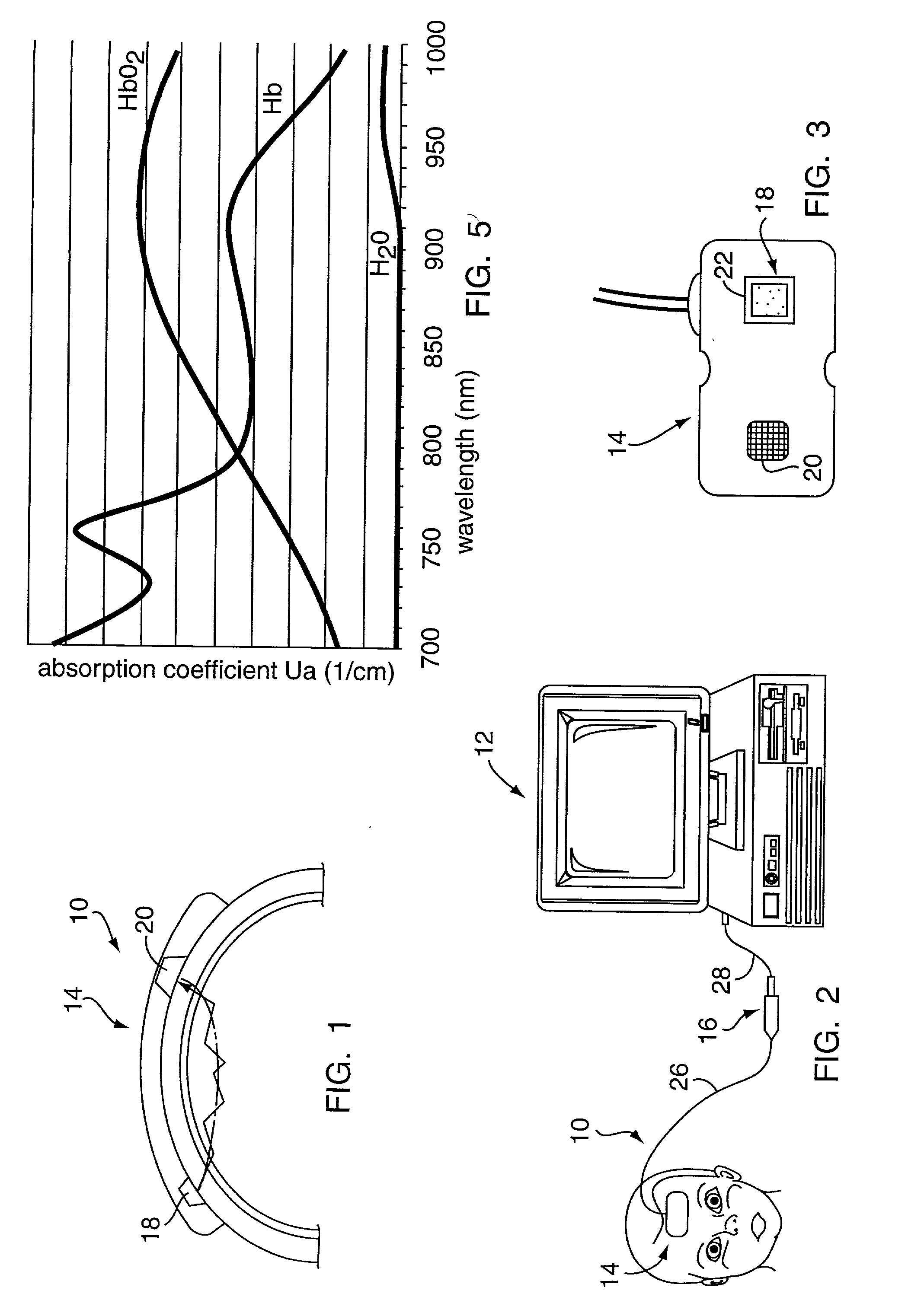
[0037] Referring to FIGS. 1-5, the preferred NIRS sensor includes a transducer portion 10 and processor portion 12. The transducer portion 10 includes an assembly housing 14 and a connector housing 16. The assembly hous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength range | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com