Azomethine compound and oily magenta ink
- Summary
- Abstract
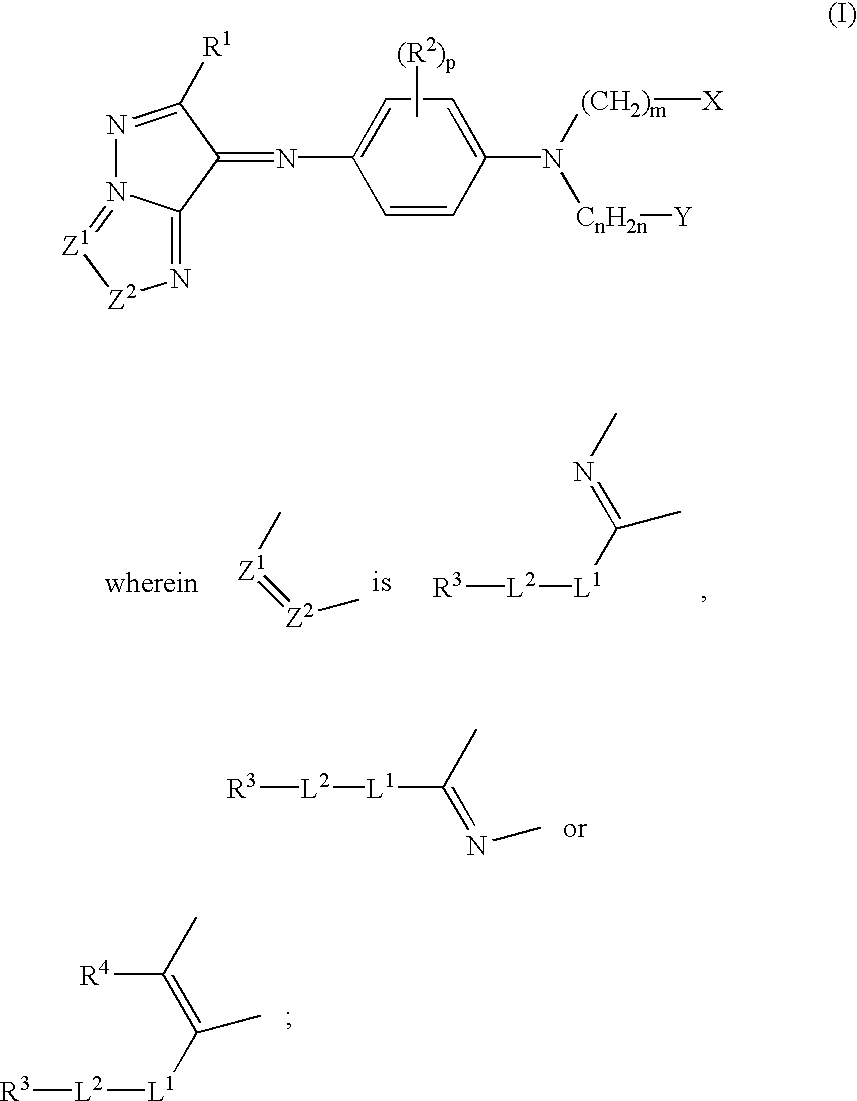
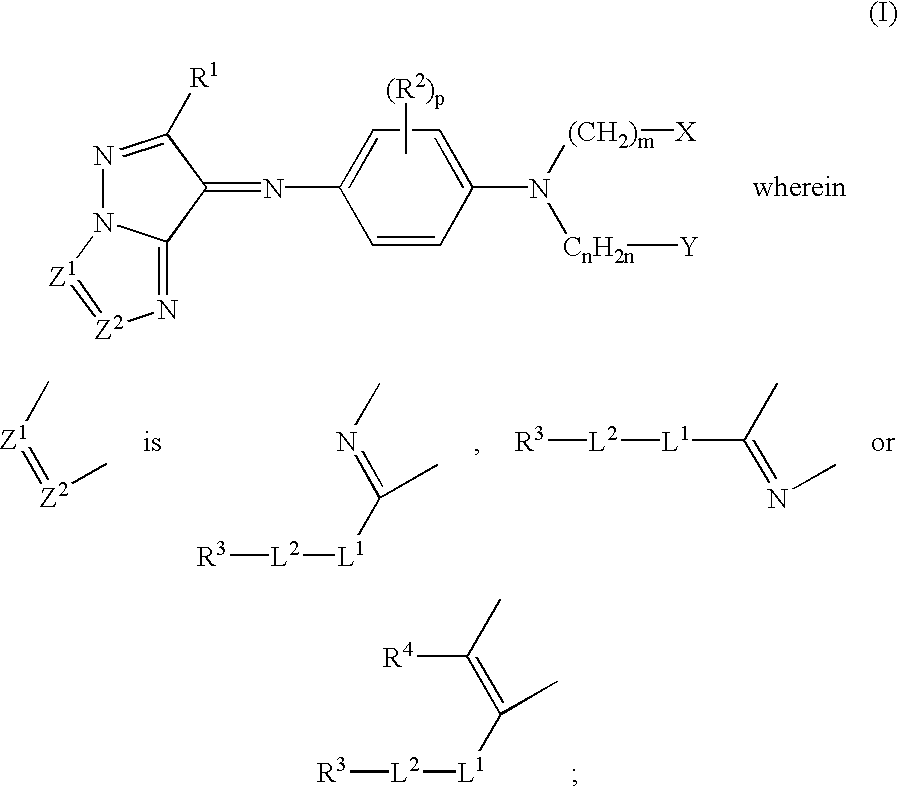
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0252] 8
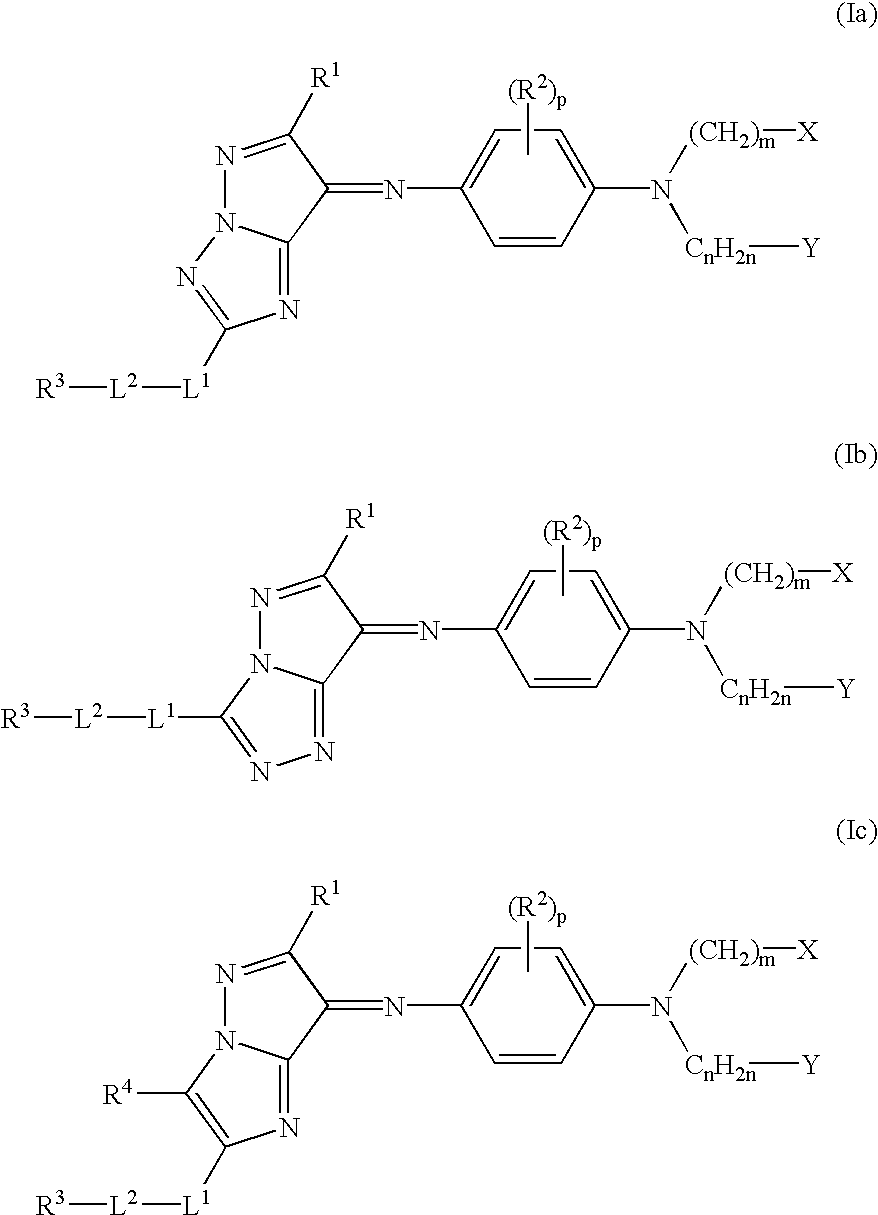
[0253] [Synthesis of azomethine compound (Ia-12)]
[0254] In 1,000 ml of ethyl acetate, 87.9 g of 2-[4-{3-(tetradecyloxycarbo- nyl) propanoylamino}phenyl]-6-tert-butyl-7-chloropyrazolo[1,5-b][1,2,4]-tr- iazole was dissolved. Independently, 400 ml of methanol and 100 g of sodium carbonate were dissolved in 750 ml of water. The prepared two solutions were mixed, and 39.1 g of 4-amino-N-butyl-N-(2-cyanoethyl) aniline was further added. The obtained solution was dropwise added for 1.5 hour to a solution in which 50.0 g of ammonium persulfate was dissolved in 300 ml of water. The resulting solution was made to react for 1 hour. After 1,000 ml of ethyl acetate and 1,000 ml of water were added, the organic phase was collected. The collected liquid was twice washed with 1,500 ml of water, and the solvent was distilled off. To the obtained oil, 300 ml of acetonitrile was added to precipitate a crystalline product. The product was collected and air-dried, and then recrystallized from 30...
example 2
[0256] 9
[0257] [Synthesis of azomethine compound (Ia-4)]
[0258] N-butylaniline and ethylacrylate were made to react to prepare N-butyl-N-(2-ethoxycarbonylethyl)aniline, which was then nitrosoated and reduced to synthesize 4-amino-N-butyl-N-(2-ethoxycarbonylethyl)aniline.
[0259] From the prepared compound, the azomethine compound (Ia-4) was synthesized in the same manner as described in Example 1.
example 3
[0260] 10
[0261] [Synthesis of azomethine compounds]
[0262] The azomethine compounds (Ia-1), (Ia-6), (Ia-13), (Ia-14) and (Ia-15) were synthesized in a similar manner to Example 1 or 2.
[0263] The melting points of the compounds (Ia-1) and (Ia-6) are lower than room temperature (i.e., they are in the form of oily liquid at room temperature). The melting point of the compound (Ia-13) was 132-133.degree. C. , the melting point of the compound (Ia-14) was 119-120.degree. C. , and the melting point of the compound (Ia-15) was 118-120.degree. C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com