Enrobed core medicament
a core medicament and enrobed technology, applied in the field of enrobed core medicaments, can solve the problems of equipment not being built and operated successfully, complex machinery required for high-volume implementation of procedures, and high cost, and achieves significant strength and resistance to breakag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
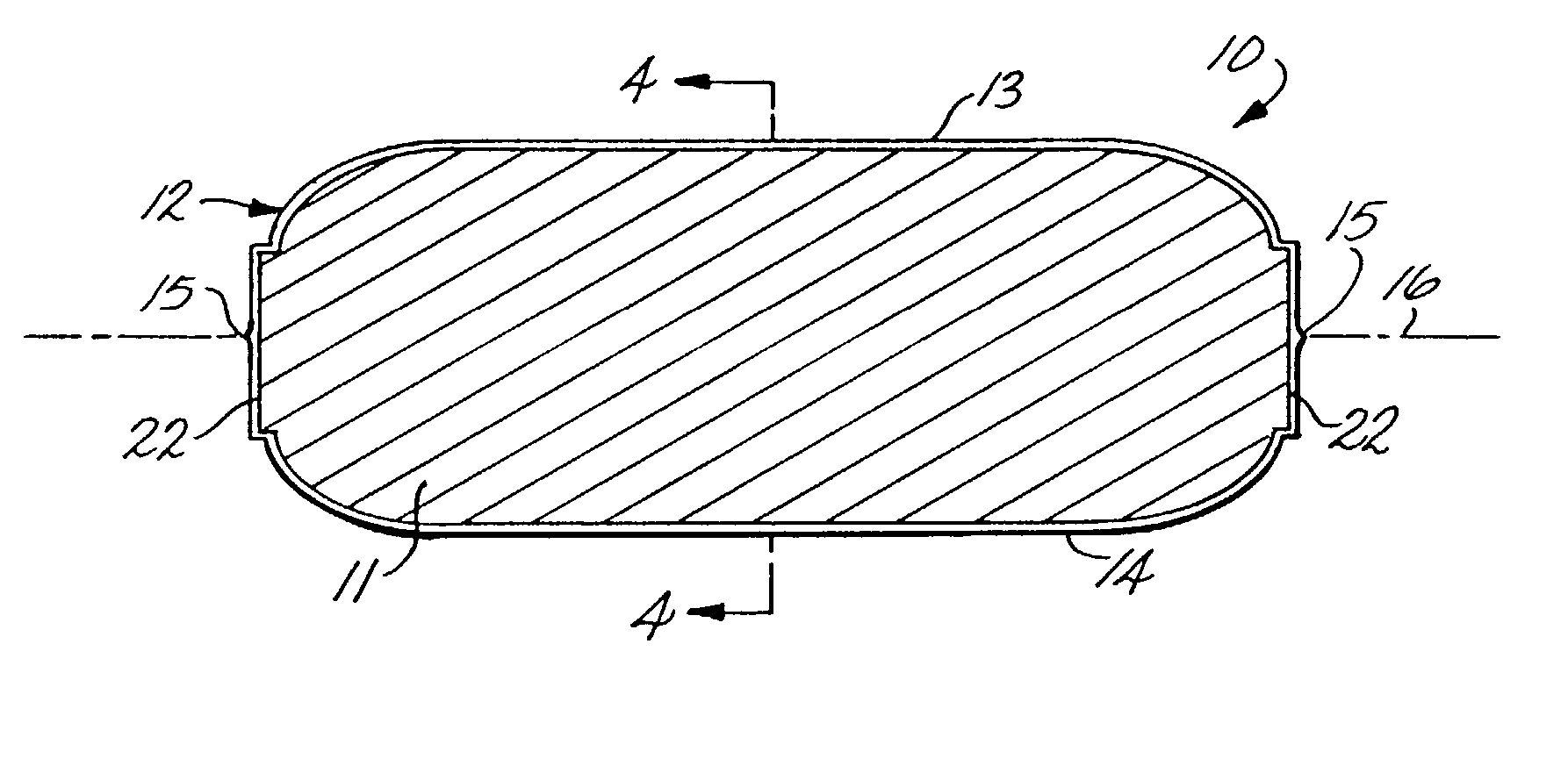
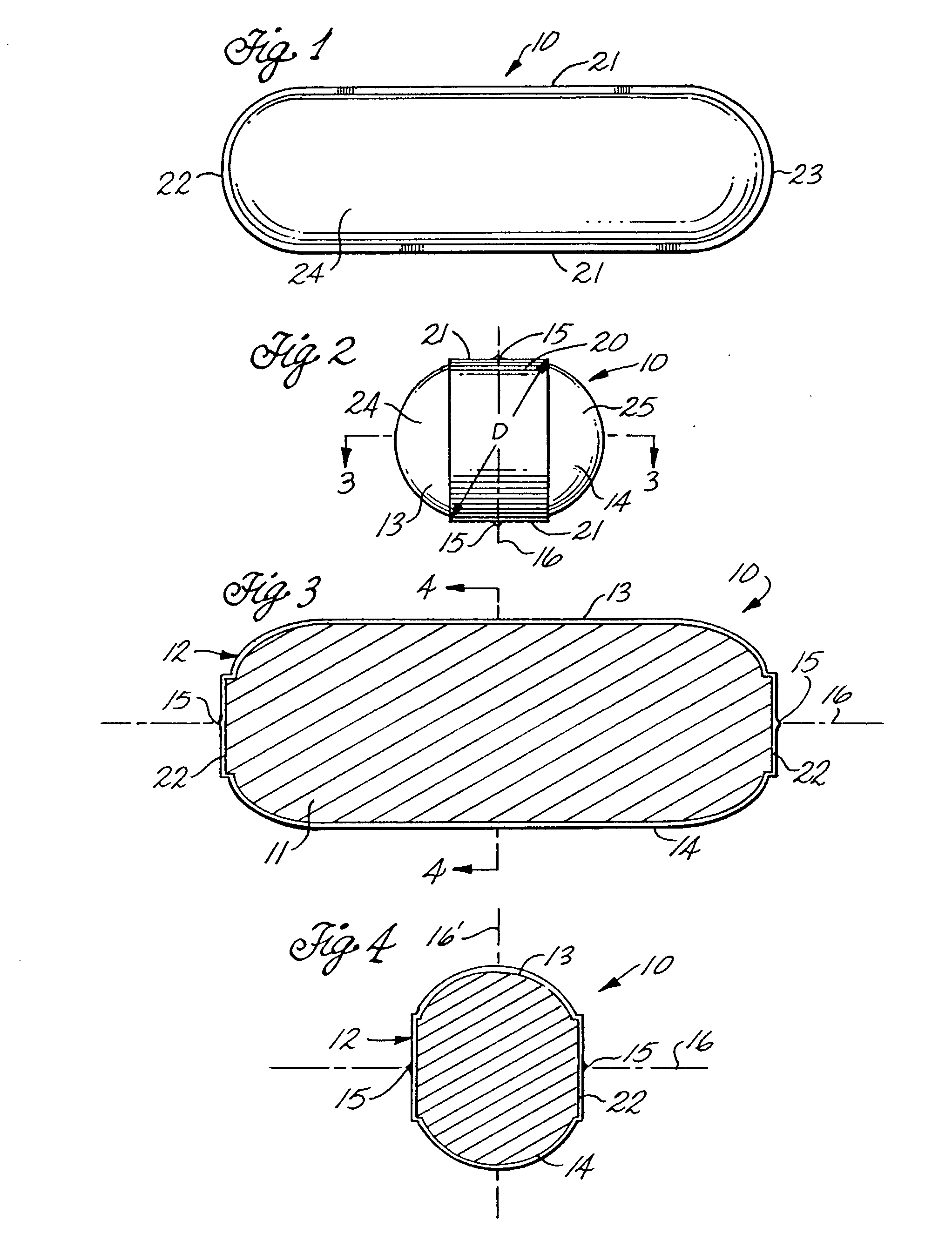
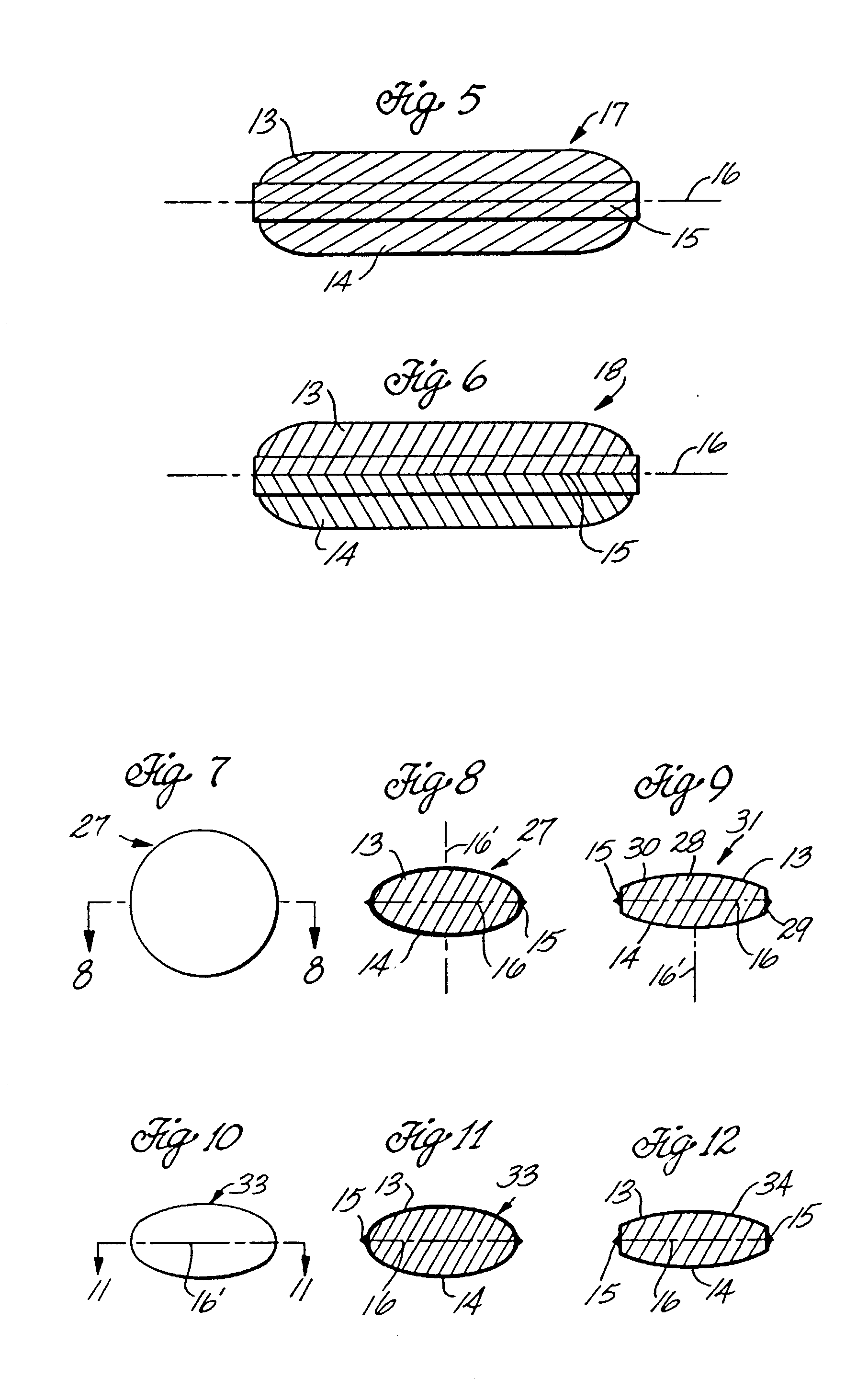
[0048] In broad terms, this invention concerns the coating of tablets, other solid dosage forms, and a variety of solids by enrobement with films of gelatin or other sealable polymers by an enrobement process which uses coacting die techniques in which the tablets or other articles to be enrobed are introduced individually between two sealable films positioned between opposing matching dies configured to cause the films to stretch and deform around each introduced article so that the films move into contact with each other, are sealed to each other and, as sealed, are severed from the film webs to provide individual film-enrobed end products. The particular product which formed the focus of the development of this invention is a tablet of caplet configuration enrobed between applied gelatin films which adhere to the solid tablet core of the product to produce a non-peelable, tamper-evident and potentially tamper-resistant gelatin coated caplet-type medicine tablet. It was found, in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com