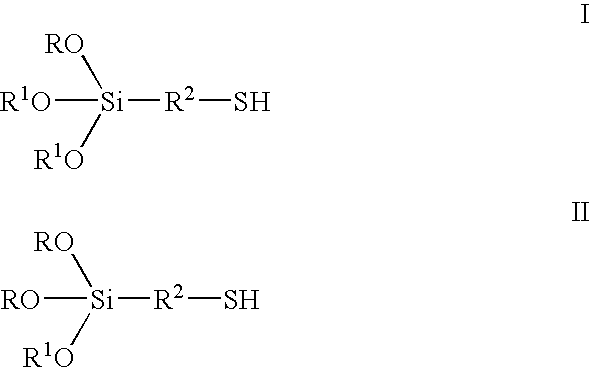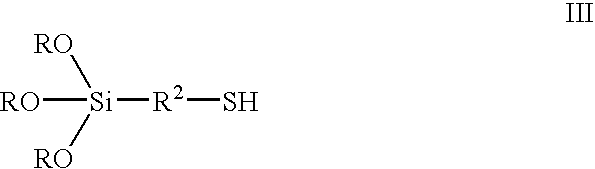Organosilicon compounds, process for their production and their use
a technology of organic silanes and compounds, applied in the field of organic silane compounds, can solve the problems of low hardness and dynamic rigidity of rubber compounds, the need to add alkyl silanes to rubber compounds, and the release of alcohol during and after bonding to fillers, etc., to achieve good dry performance, improve wet skid resistance, and improve the effect of hardness and dynamic rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0066] A mixture consisting of 286.1 g 3-mercaptopropyl triethoxysilane (formula III where R=CH.sub.2CH.sub.3, R.sup.2=--CH.sub.2CH.sub.2CH.sub.2---), 313.1 g dodecanol (R.sup.1=--C.sub.12H.sub.25) and 154.4 g 1-tetradecanol (R.sup.1 =--C.sub.14H.sub.29) is heated with 140 .mu.l tetra-n-butyl orthotitanate to 110.degree. C. in a 1-litre flask in a rotary evaporator and ethanol that is produced is distilled off over 4 h in vacuo at 40 mbar. 636.86 g (99.0%) of a colorless liquid having formula I, where R=--CH.sub.2CH.sub.3, R.sup.1 =--C.sub.12.6H.sub.26.2, R.sup.2=--CH.sub.2CH.sub.2CH.sub.2--), is obtained.
example 2
[0067] Production and analysis of the rubber compounds according to the invention The formulation used for the rubber compounds is set out in Table 1 below. The unit phr denotes contents by weight, relative to 100 parts of the crude rubber used. The organosilicon compound according to the invention is added in equimolar quantities to 3-mercaptopropyl triethoxysilane relative to silicon. The general process for the production of rubber compounds and vulcanizates thereof is described in the book: "Rubber Technology Handbook", W. Hofmann, Hanser Verlag 1994.
1 TABLE 1 Compound 1 Compound 2 Reference Reference Compound 3 Stage 1 Buna VSL 5025-1 96 96 96 Buna CB 24 30 30 30 Ultrasil 7000 GR 80 80 80 3-mercaptopropyl 2.4 -- --triethoxysilane VP Si 208 2.5 -- --Organosilicon compound -- 5.7 --according to example 10 DE 10137809.2 Organosilicon compound -- -- 5.4 according to example 1 ZnO 2 2 2 Stearic acid 2 2 2 Naftolen 10 10 10 Vulkanox 4020 1.5 1.5 1.5 Protektor G35P 1 1 1 Stage 2 Batch...
example 3
[0076] 268.08 g 3-mercaptopropyl triethoxysilane and a mixture consisting of 313.05 g 1-dodecanol and 154.36 g 1 tetradecanol are placed in a 1-litre three-necked flask with distillation attachment at room temperature and 1.0 g toluene-p-sulfonic acid monohydrate is added. The solution is heated to 120.degree. C. The ethanol that is produced is continuously removed by distillation. Distillation is then performed in a rotary evaporator in vacuo at 80.degree. C. and 20 mbar. 638.7 g (99%) of a colorless liquid according to formula I is obtained, where R=--CH.sub.2CH.sub.3, R.sup.1=mixture of --C.sub.12H.sub.25 and --C.sub.14H.sub.29 in the ratio 2:1 and R.sup.2=--CH.sub.2CH.sub.2CH.sub.-2--.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com