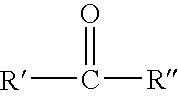
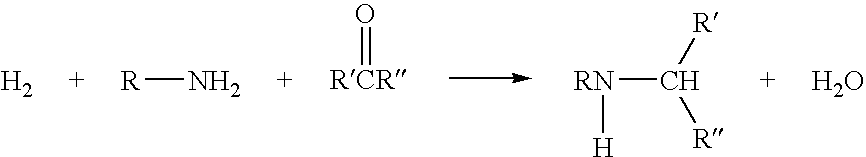Preparation of secondary amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0020] Preparation of N-isopropyl-N',N'-dimethyl-1,3-propylenediamine
[0021] The procedure of Example 1 was repeated, with the exception that the nickel catalyst used previously was replaced by a 1% palladium on carbon catalyst (Engelhard) and the reaction temperature was reduced to 120.degree. C. The effluent was analyzed and gave about 97% yield of N-isopropyl-N',N'-dimethyl-1,3-propylenediamine. No significant amounts of di-isopropyl DMAPA or coupling products were detected. The effluent was distilled to give 99.5% pure N-isopropyl-N',N'-dimethyl-1,3-propylene-diamine.
example 3
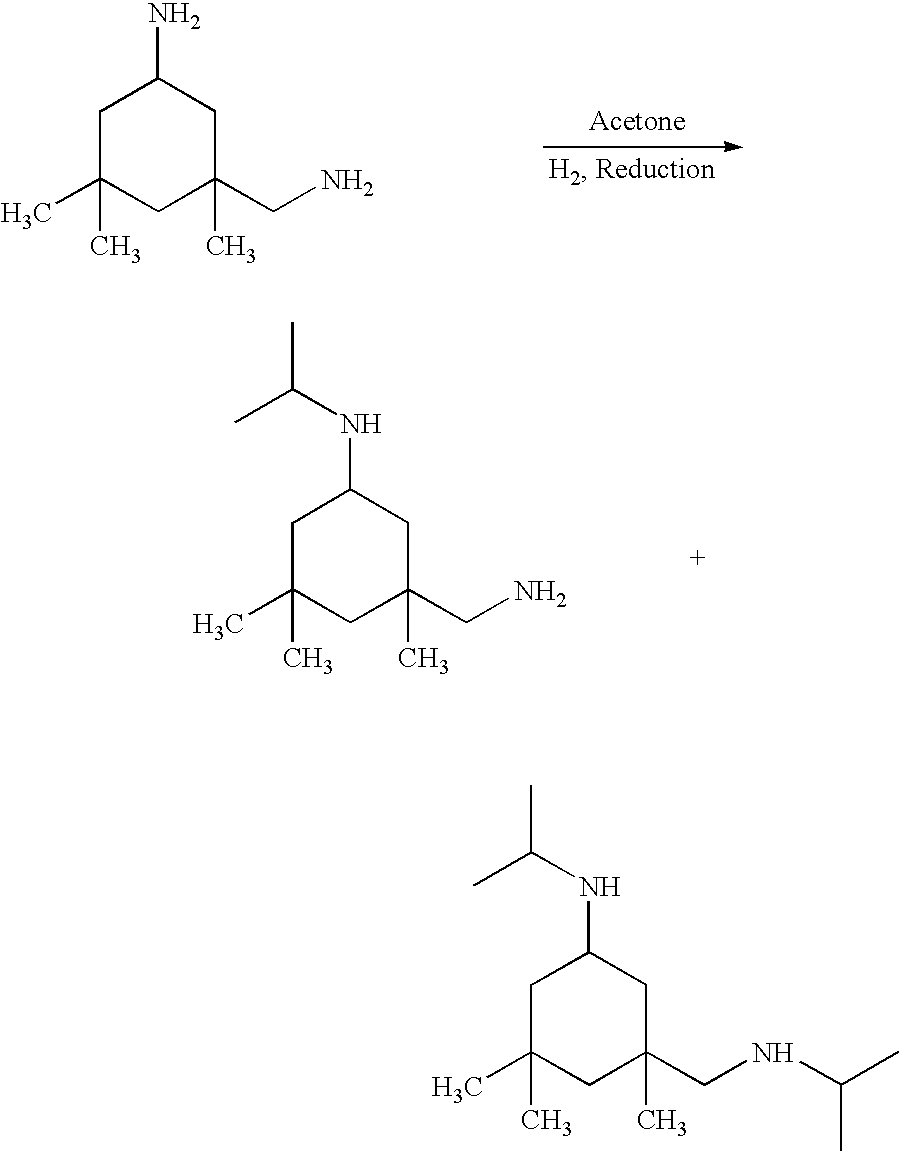
[0022] Preparation of N,N'-Diisopropylisophorone Diamine
[0023] About 300 g per hour of isophorone diamine and 450 g per hour of acetone were fed upflow into a 600 cc packed-bed reactor filled with a nickel catalyst as described in example 1 above. Hydrogen was fed at about 100% in excess. The reaction was conducted at 2000 psig and 140.degree. C. Lights were stripped out of the reactor effluent under reduce pressure. The resulting product was analyzed to contain 8.78 meq / g of total amine and 3.557 meq / g of primary amine. This result indicates that a significant amount (40.5%) of primary amine group was not alkylated. Also, GC analysis showed about 2.00% of "heavies" were present. In other words, amine coupling had occurred.
example 4
[0024] Preparation of N,N'-Diisopropylisophorone Diamine
[0025] A 200 cc DOWTHERM.RTM. heated stainless steel tubular upflow reactor which has an inside diameter of 0.815" and a thermostat fabricated from 0.25" OD tubing extend upwardly into the reactor was used. The reactor was filled with a 1.0% palladium on carbon catalyst (Engelhard). About 100 g per hour of isophorone diamine and 135 g per hour of acetone was fed into the tubular reactor, simultaneously, along with hydrogen at about 100% in excess. The reaction was conducted at 2000 psig and 150.degree. C. Lights were stripped out of the reactor effluent under reduce pressure. The resulting product was analyzed to contain 7.89 meq / g of total amine, 0.07 meq / g of primary amine, and 0.04 meq / g of tertiary amine. GC analysis also showed no evidence of coupling. This result indicates, with Pd / C catalyst, high conversion and selectivity were achieved.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com