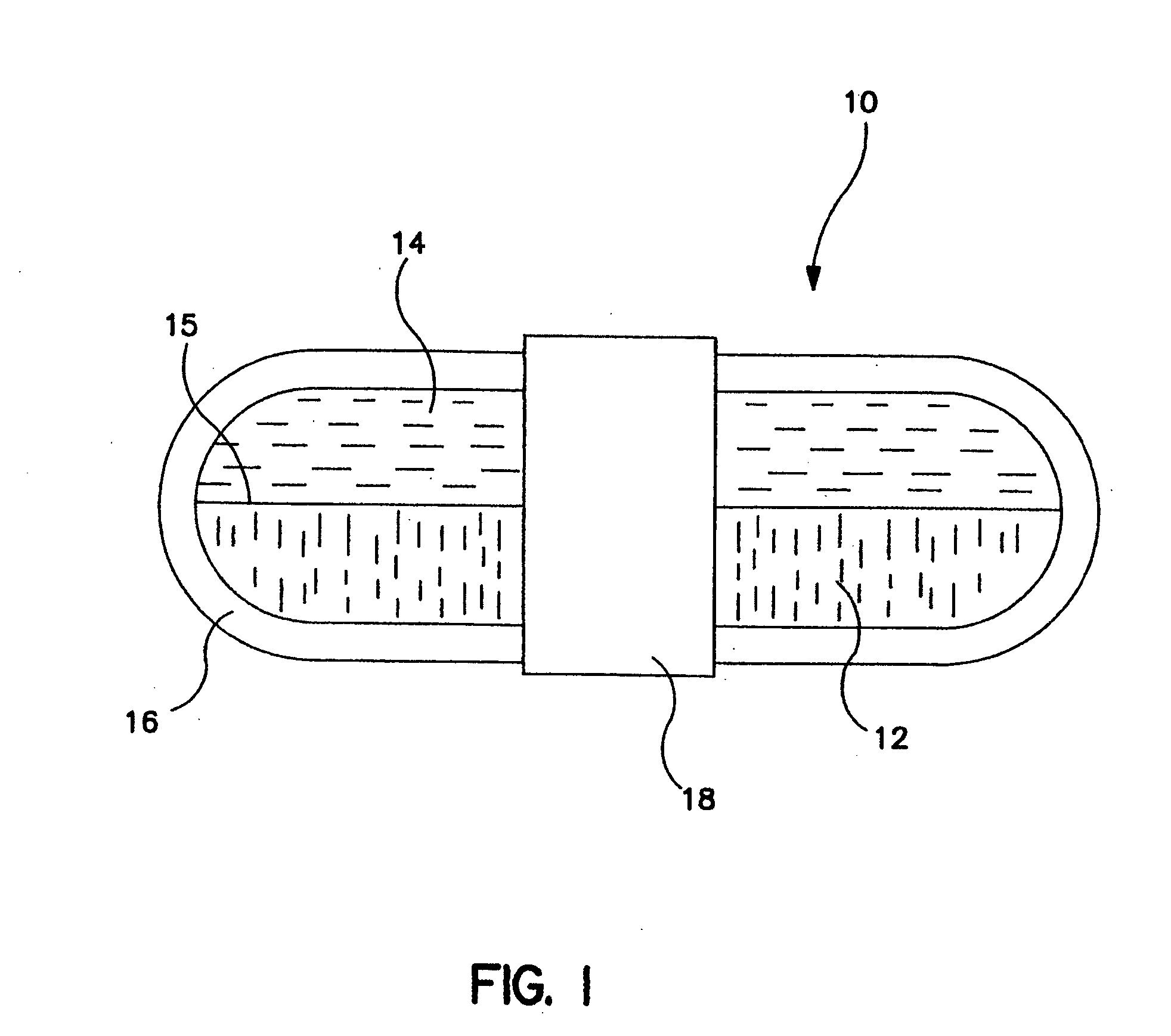
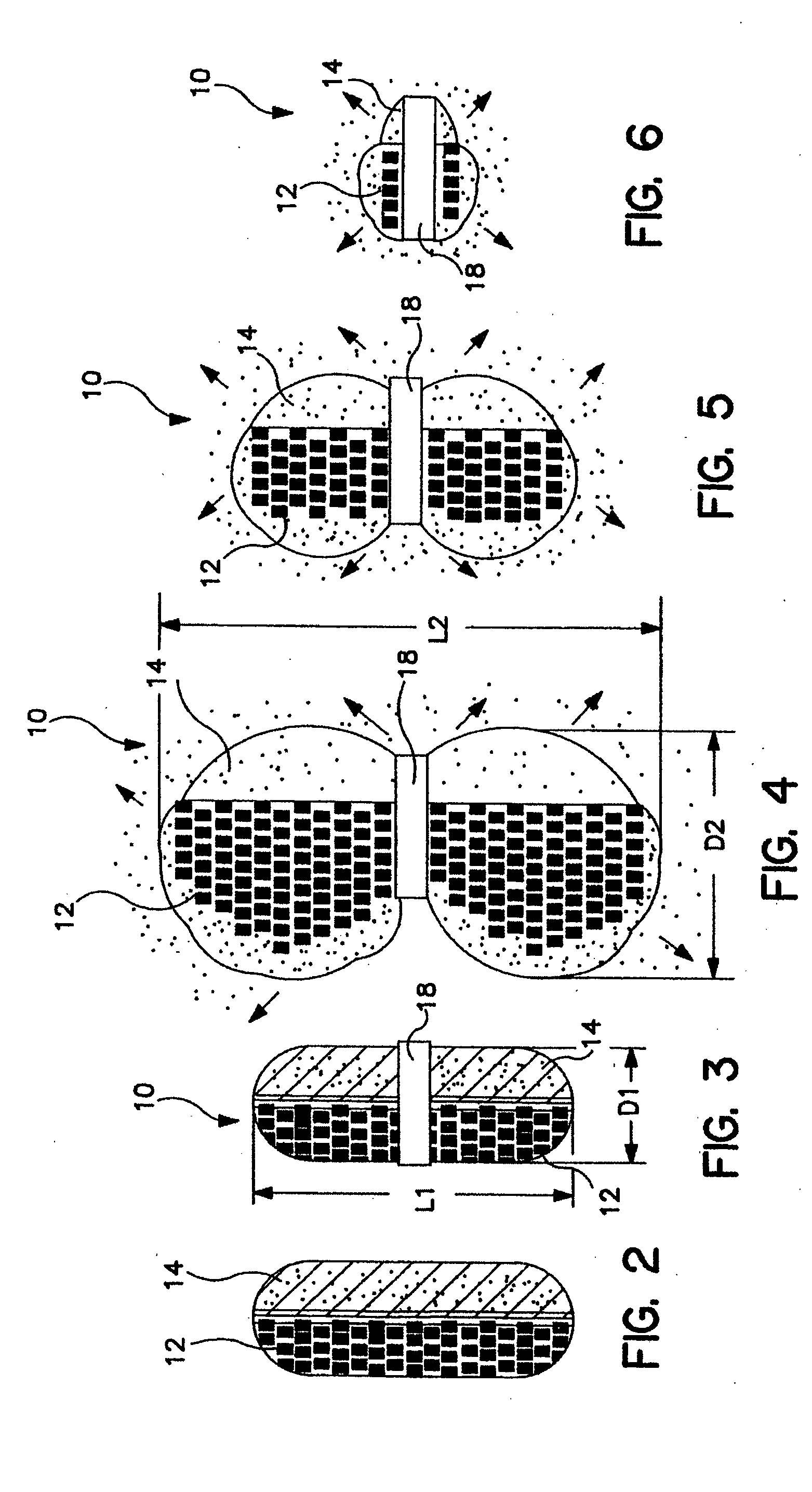
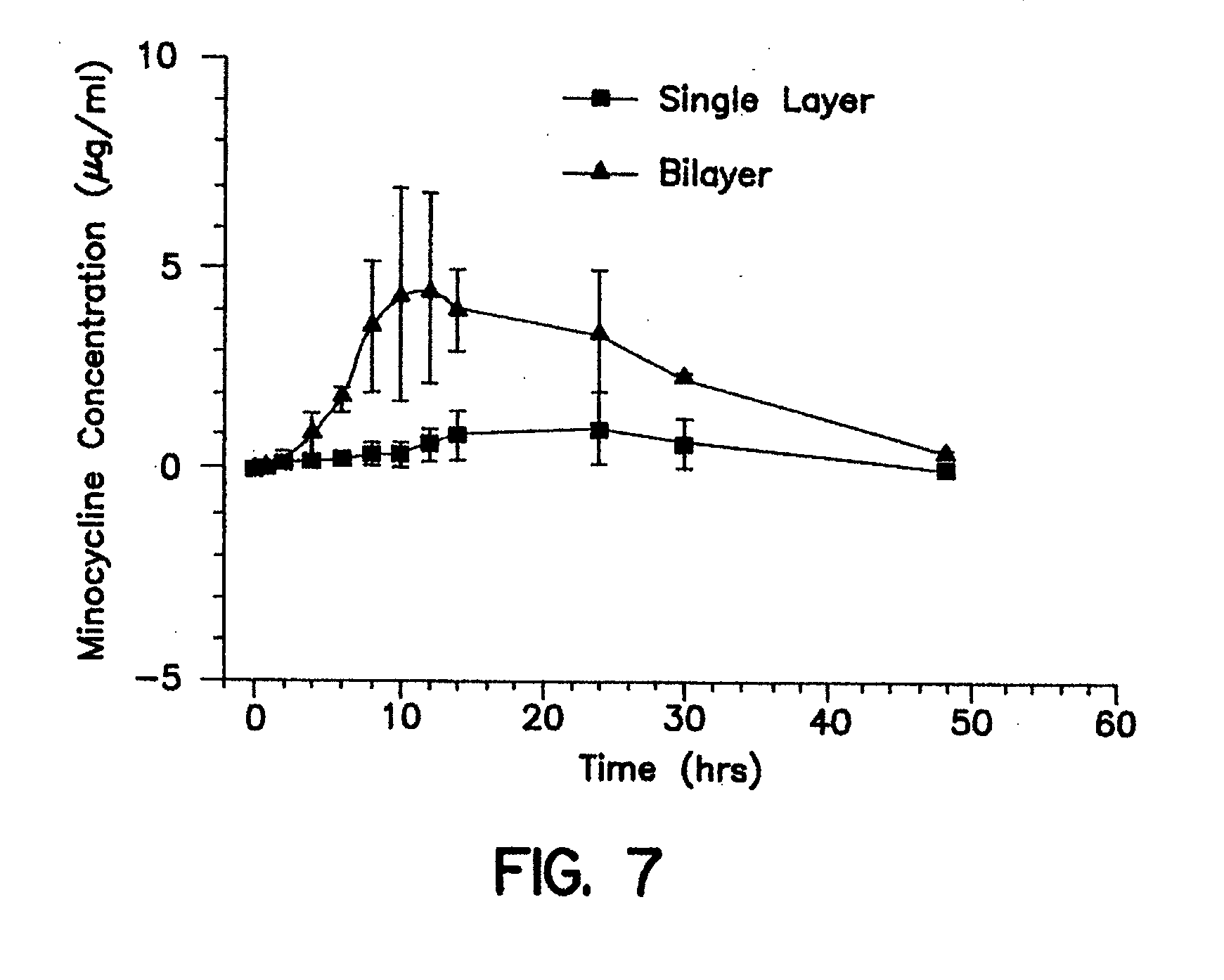
Gastric retention dosage form having multiple layers
a dosage form and active agent technology, applied in the direction of pharmaceutical active ingredients, pill delivery, medical preparations, etc., can solve the problems of inability to generally leave the stomach of non-digested material, inconvenient use for users, and short window for absorption of active agents in the small intestine to provide a desired therapeutic effect, etc., to achieve the effect of prolonging the delivery of active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation 1
An example of a active agent which requires frequent dosing is acyclovir. A typical dosing regimen for this antiviral active agent is five doses per day administered every four hours. A dosage form in accordance with this invention for twice daily dosing of acyclovir is formulated according to the following procedures. The dosage form is retained in the stomach and releases acyclovir over a prolonged period of time.
22.5 Grams of acyclovir and 3.6 grams of the gel-forming polymer polyethylene oxide, having a number average molecular weight of approximately 200,000 grams per mole, are separately screened through a mesh having 40 wires per inch. The polyethylene oxide is supplied under the trade name Polyox® grade WSR N80 as manufactured by Union Carbide Corporation, Danbury, Conn. The sized active agent and polymer are dry mixed. Then, 3.75 grams of a hydroattractant water-insoluble polymer, hydroxypropyl cellulose having a hydroxypropyl content of 10-13 weight percent and an avera...
example 1 (
Banded devices fabricated in Preparation 1 are assayed for dimensional changes and release of active agent as follows:
A banded dosage form is placed in a beaker of simulated gastric fluid, as specified in U.S. Pharmacopedia / National Formulary 23 / 18, having a pH of approximately 1.4 and a maintained temperature of 37° C. After one hour, the device is removed and measured for dimensional change in length and diameter. The swollen device will have the general appearance of the dosage form shown in FIG. 4.
Samples of the dosage form are tested for release of active agent by shaking at prescribed conditions in an aqueous media simulating the media in the upper gastrointestinal tract. Each dosage form is first placed in a cylindrical, slotted basket having inside diameter of 15 mm and inside length of 52 mm. Each basket has eight slots and each slot is 1-2 mm wide and 52 mm long and positioned lengthwise along the length of the basket. The basket containing the dosage form is th...
example 2
Dosage forms of this invention containing the antiviral drug ganciclovir are prepared in accordance with the procedures of Preparation 1. The dosage forms prepared are retained in the stomach and release ganciclovir over a prolonged period of time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com